Indexed In
- Open J Gate
- Genamics JournalSeek
- CiteFactor
- Cosmos IF
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Directory of Abstract Indexing for Journals
- OCLC- WorldCat
- Proquest Summons
- Scholarsteer
- ROAD
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2024) Volume 16, Issue 1
Comparison between Two Extraction Methods of Plant Lectins from Ruta graveolens L. and their Partial Characterization
Amairany Torres Alvarez, Laura Flores Garcíaa, Ernesto Mendoza* and Catalina Machuca RodríguezaReceived: 06-Jan-2024, Manuscript No. BLM-24-24556; Editor assigned: 08-Jan-2024, Pre QC No. BLM-24-24556(PQ); Reviewed: 22-Jan-2024, QC No. BLM-24-24556; Revised: 29-Jan-2024, Manuscript No. BLM-24-24556(R); Published: 07-Feb-2024, DOI: 10.35248/0974-8369.23.16.636
Abstract
Background: Ruta graveolens L. is a plant wich has several uses among different communities, such as an anti- inflammatory and antitumor, related to the plant lectins available in their different tissues.
Methods: Extraction of these molecules is achieved through diverse extraction methods, without knowledge about which one is better to preserve their native structure. In this study two well-documented methods are compared.
Results: Lectin-enriched extracts were obtained out of different R. graveolens L. organs, and those were purified trough NaCl salt extraction and Tris-HCl extraction methods. Additionally, several eluents were used in the affinity chromatography method.
Conclusion: Lectin-like proteins with affinity to several carbohydrates were found. The NaCl extraction method was more efficient at extracting lectin-like proteins than the Tris-HCl method, comparing to the total-protein extract. These results suggest that Tris-HCl buffer is more efficient to extract other proteins.
Keywords
Plant lectin, Extraction, R. graveolens L, Partial characterization.
Introduction
Ruta graveolens L. (Rue) is a plant from the Rutacea family. Due to its several medicinal properties, nowadays it’s became more relevant as a cancer treatment candidate as a chemotherapeutic, antineoplastic and clastogenic agent [1,2] which has been related to lectin-like plant proteins [3,4].
Plant lectins are non-immune glycoproteins which can bind to carbohydrates in a specific and reversible way and have at least two binding-sites which allows them to bind to a specific sugar and to a glycosylated molecule-1 [4-6], but lacking enzymatic activity [7-9].
This kind of molecules are usually found in cotyledon and endosperm, adding up to 2%-10% of the total protean portion, but could also be found in tubers, bulbs, and rhizomes, as these could fulfill a defensive function, as insecticide, antimicrobe, among others [5,10].
Plant lectins can be found in vegetative tissues, such as roots, leaves, stem, cortex, flowers, fruits, phloem sap, latex and nectar [3,11,12].
Those molecules are made out of a polypeptide chain which could be joint to 1 to 15 monosaccharide residues, constituted of at least two sugars, such as D-mannose, D-galactose, D-glucose, L-fucose, N-acetyl-D-glucosamine, N-acetyl-D-galactosamine, salicylic acid, glucosamine and galactosamine [13].
Plant lectin main extraction methods are based on the salting out method, which promotes the decrease of the protein’s solubility increasing the ionic force of the solution, thus decreasing the protein-water interaction through the loss of the solvation layer and inducing its precipitation. These are mediated through acid- base groups into the protean structure, allowing solubility changes depending on the ionic force [14], which is constituted by the concentration and the electric charges in the anions and cations donated by the salt, inducing the purification of certain proteins.
Even though plant lectin studies have generated relevant information related to its biological properties, there hasn’t been determined which saline extraction method is more suitable to obtain a better lectin-like protein yield without sacrificing its structure and biological functions.
The main objective of this work is to compare the two main saline extraction methods used to obtain lectin-like proteins from Ruta graveolens L., partially characterizing the obtained proteins.
Materials and Methods
Sample collection
Ruta graveolens L. was obtained from the Xochimilco plant market, at Mexico City. Taxonomic determination was made using a vascular plant dichotomous key [15]. Extracts were obtained from 100 grams of each structure, leaves, stem and roots.
Salt extraction
Was conducted according to Villarrubia et al., [16] in 1995, and Torres et al., [17] method, modified. Plant tissues were macerated in a mortar with 5% NaCl solution at 4°C, with a 3:1 proportion, 50 mM Tris-HCl solution in a 4:1 proportion and PBS in a 5:1 proportion, the last being used only to obtain proteins from the root. Macerates were let to sit for 24 hours at 4°C. Vegetal material excess was removed through sterile gauze and #41 Whatman paper. Extracts were centrifuged at 3000 rpm for 20 minutes.
The precipitated was discarded and the supernatant was dialyzed using a 3 KDa cellulose membrane against a 0.1 M pH 8 ammonium bicarbonate buffer (NH4HCO3) with constant agitation, changing the medium each 12 hours. A qualitative evaluation was performed using a 0.01 N silver nitrate solution (AgNO3) 0.01 N to determine the NaCl absence.
Affinity chromatography
Dialyzed fractions were demi purified through packed columns using a G60 silica gel matrix. Samples were made pass through the column and then retained proteins were eluted with 0.1 M solutions of different sugars, such as Glucose (Glu), Arabinose (Ara), Galactose (Gal), Lactose (Lac), Maltose (Mal), Mannitol (Man), Saccharose (Sac), Sorbitol (Sr) and Fructose (Fru). Each fraction was dialyzed against a 0.1 M pH 8 ammonium bicarbonate buffer (NH4HCO3) until carbohydrate elimination assessing carbohydrate presence through the Dubois method.
Protein quantification
Protein quantification was performed in each demi purification step through biuret an UV methods [18].
Carbohyrate quantification
Total carbohydrate quantification was performed through Dubois method, using 100 μl of each sample, adding 100 μl of 5% phenol. After 15 minutes 3 ml of sulphuric acid were added, letting it sit for 30 minutes to allow the colors to develop. Samples’ absorbance was assessed at 540 nm. Reducing carbohydrates were assessed trough the Miller method. 500 μl of each sample were used, adding up to 1000 μl using distilled water. 500 μl of DNS reactive were added, and the samples were double-boiled for 5 minutes.
Reaction was stopped through a thermic shock using 4°C water. 5 ml of distilled water were added, letting them sit for 15 minutes and absorbance was assessed at 540 nm. Non-reducing sugar concentration was asessed throug a substraction of reducing sugars from total sugars concentration.
SDS-PAGE electrophoresis
Proteins were quantified trough the Bradford method. Electrophoresis was performed according to the Laemmeli (1970) method, using a 12% polyacrylamide gel with an electrophoretic displacement of 90 V. Gel development was performed using the silver staining [19].
Hemagglutination assessment
Intact human erythrocytes corresponding to the ABO+ system were used. Red blood cells were collected using EDTAK2 Vacutainer tubes; and were prepared according to the method proposed by Rodríguez et al., [19,20] centrifuging at 3000 rpm for 15 minutes, discarding the supernatant and resuspending the erythrocytes in 10 pH 7.4, 295 mOsm/kg PBS volumes thrice. Finally, erythrocytes were resuspended in a 3% PBS dilution. To assess the hemagglutination dynamic, three seral dilutions of the lectin- like protein extracts were performed, adding 50 μl of 3% human erythrocytes, incubating for 1, 2, 3, 4, 5…24 hours.
Results
Comparison of the lectin-like protein of Ruta graveolens yield through different extraction methods.
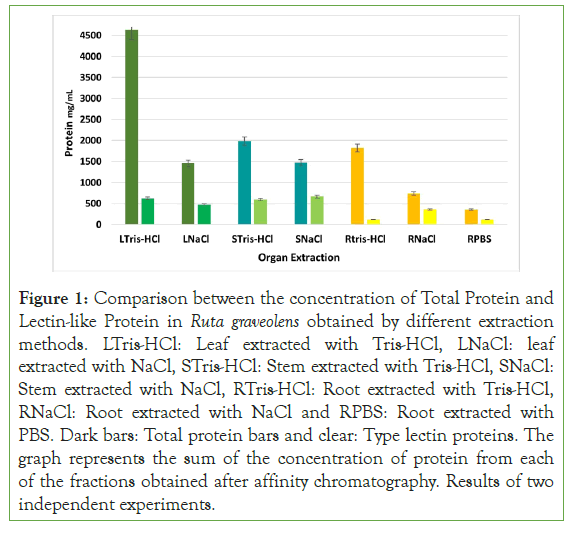
The bigger total protein yield was obtained out of the leaves extract, followed by the stem extract and finally, the root extract. Comparing both extraction methods, the Tris-HCl extraction method, characterized for having low ionic force, allow to get a yield of 4633.6 mg in the leaf extract, 1987.2 mg in the steam extract and an 1821.83 mg in root extract for each 100 g; while through NaCl extraction, characterized for having a high ionic force a yield of 1459.2 mg in leaf extract, 1472.64 mg in stem extract, and 737.37 mg in root extract was obtained. It can be observed that Tris-HCl extraction method allows obtaining twice and thrice the total protein concentration compared to the NaCl and PBS extraction method.
Regarding to the plant lectin-like proteins, the NaCl extraction method allowed to obtain a bigger concentration, with 32.51% in leaf extract (474.48 mg/ml), 45.31% in stem extract (667.35 mg/ ml) and 48.75% in root extract (359.48 mg/ml), compared to Tris- HCl extraction method, obtaining a yield of 13.49% from leaves, (625.41 mg/ml), 30.03% from stems (596.78 mg/ml) and 6.38% from roots (116.29 mg/ml) (Figure 1 and Figure S1).
Figure 1: Comparison between the concentration of Total Protein and Lectin-like Protein in Ruta graveolens obtained by different extraction methods. LTris-HCl: Leaf extracted with Tris-HCl, LNaCl: leaf extracted with NaCl, STris-HCl: Stem extracted with Tris-HCl, SNaCl: Stem extracted with NaCl, RTris-HCl: Root extracted with Tris-HCl, RNaCl: Root extracted with NaCl and RPBS: Root extracted with PBS. Dark bars: Total protein bars and clear: Type lectin proteins. The graph represents the sum of the concentration of protein from each of the fractions obtained after affinity chromatography. Results of two independent experiments.
After the demi purification processes and using different eluents enriched lectin-like R. graveolens protein fractions were obtained out of its different organs (Figure S2) (Tables S1 and S2).
Determination of carbohydrate concentration present in fractions enriched with plant lectin-like proteins of R. graveolens obtained by different extraction methods
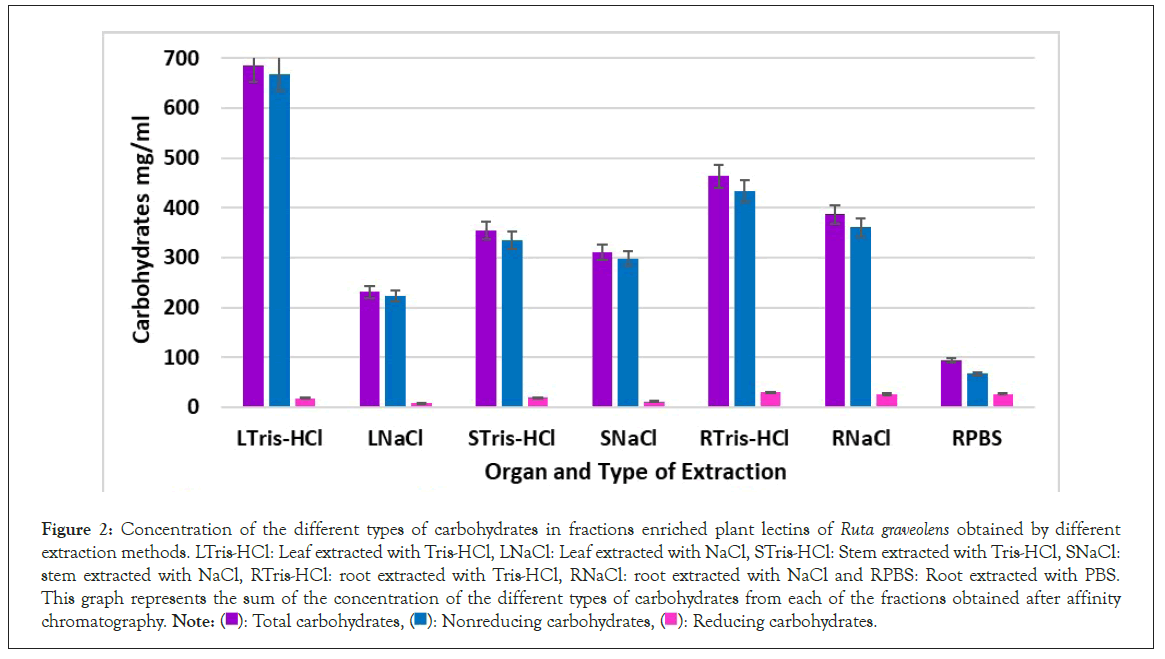
Carbohydrates in the protean fraction were assessed as part of the biochemical characterization. In Figure 2 the average concentration of different carbohydrates in leaf fractions extracted with NaCl was 230.85 mg/ml Total Sugars (TS), 222.71 mg/ml Non Reducing Sugars (NRS) and 8.14 mg/ml Reducing Sugars (RS), corresponding to 32.7% out of the total mass.
Figure 2: Concentration of the different types of carbohydrates in fractions enriched plant lectins of Ruta graveolens obtained by different extraction methods. LTris-HCl: Leaf extracted with Tris-HCl, LNaCl: Leaf extracted with NaCl, STris-HCl: Stem extracted with Tris-HCl, SNaCl: stem extracted with NaCl, RTris-HCl: root extracted with Tris-HCl, RNaCl: root extracted with NaCl and RPBS: Root extracted with PBS. This graph represents the sum of the concentration of the different types of carbohydrates from each of the fractions obtained after affinity chromatography. Note: ( ): Total carbohydrates, (
): Total carbohydrates, ( ): Nonreducing carbohydrates, (
): Nonreducing carbohydrates, ( ): Reducing carbohydrates.
): Reducing carbohydrates.
Regarding to Tris-HCl extraction method, the average concentration of different carbohydrates in leaf fractions was 685.94 mg/ml TS, 668.36 mg/ml NRS and 17.58 mg/ml RS, corresponding to 52.3% out of the total mass.
Stem fractions extracted with NaCl, average concentration of TS was 309.91 mg/ml, 298.48 mg/ml of NRS and 11.49/ml of RS, corresponding to 31.7% of the total mass. In stem fractions extracted with Tris-HCl, average concentration of TS was 354.09 mg/ml, 334.8 mg/ml of NRS and 19.29 mg/ml of RS, corresponding to 37.23% of total mass. In root extracts, average concentration of TS was 386.43 mg/ml, 360.36 mg/ml of NRS and 26.07 mg/ml of RS when extracted with NaCl, corresponding to 54.73% of total mass; Tris-HCl extracted-samples have an average TS concentration of 463.2 mg/ml, 433.84 mg/ml of NRS and 29.35 mg/ml of Rsadding up to 81.75% of the total mass. Through PBS extraction, average TS was 94.195 mg/ml, 67.525 mg/ml of NRS and 26.67 mg/ml RS, corresponding to 47.25% of the total mass.
In Figures S3A-S3C carbohydrate concentration of lectin-like protein enriched extracts from leaf, stem and roots, respectively are shown.
Determination of quantity and molecular weight of plant lectin-like proteins in Ruta graveolens obtained by different extraction methods
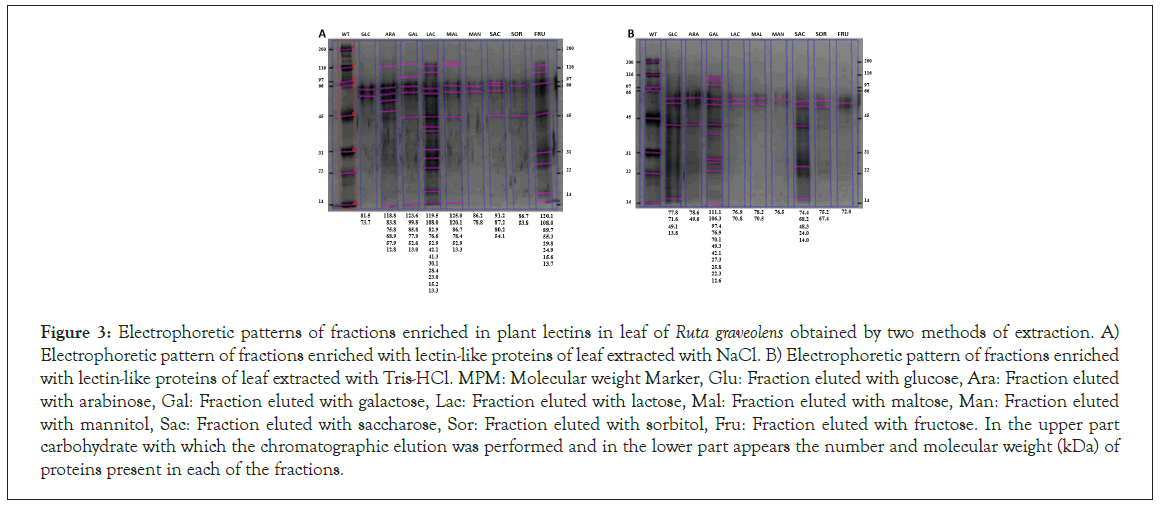
In Figure 3 is shown the protean band pattern of each leaf fraction obtained with the NaCl extraction method, in a range from 12 to 125 kDa, being arabinose, galactose, lactose, maltose, saccharose y fructose the fractions that present the most protic diversity, contrary to the Tris-HCl leaf extract electrophoretic profile, shown in F 3B, where most fractions present 2 to 4 bands, except from galactose, which presents 11 bands, ranging from 12 to 112 kDa.
Figure 3: Electrophoretic patterns of fractions enriched in plant lectins in leaf of Ruta graveolens obtained by two methods of extraction. A) Electrophoretic pattern of fractions enriched with lectin-like proteins of leaf extracted with NaCl. B) Electrophoretic pattern of fractions enriched with lectin-like proteins of leaf extracted with Tris-HCl. MPM: Molecular weight Marker, Glu: Fraction eluted with glucose, Ara: Fraction eluted with arabinose, Gal: Fraction eluted with galactose, Lac: Fraction eluted with lactose, Mal: Fraction eluted with maltose, Man: Fraction eluted with mannitol, Sac: Fraction eluted with saccharose, Sor: Fraction eluted with sorbitol, Fru: Fraction eluted with fructose. In the upper part carbohydrate with which the chromatographic elution was performed and in the lower part appears the number and molecular weight (kDa) of proteins present in each of the fractions.
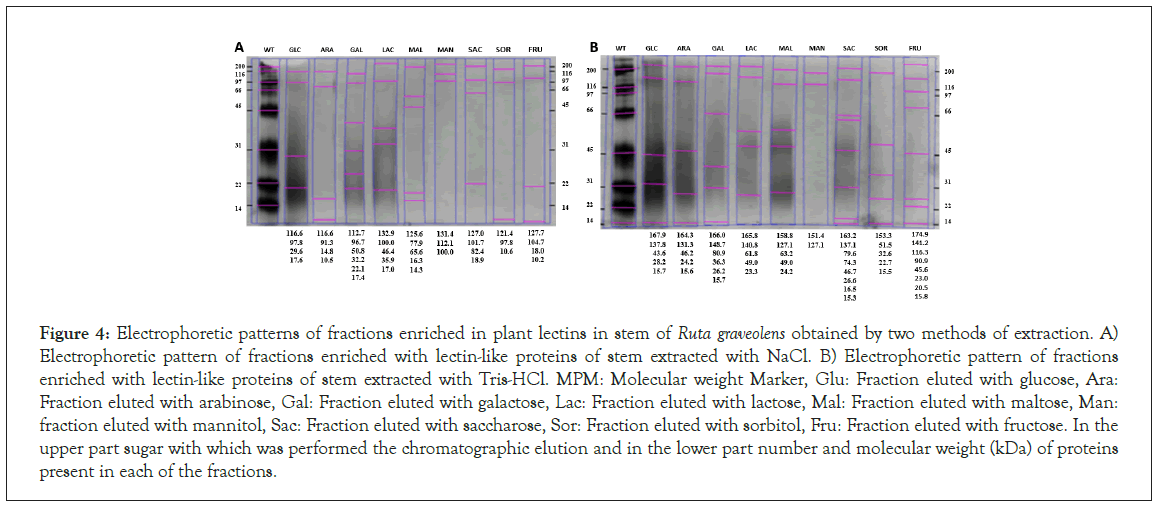
Figure 4 shows the electrophoretic pattern of stem lectin-like proteins extracted through the NaCl method, ranging from 10.5 to 133 kDa, being the galactose fraction the one with bigger protein diversity.
Figure 4: Electrophoretic patterns of fractions enriched in plant lectins in stem of Ruta graveolens obtained by two methods of extraction. A) Electrophoretic pattern of fractions enriched with lectin-like proteins of stem extracted with NaCl. B) Electrophoretic pattern of fractions enriched with lectin-like proteins of stem extracted with Tris-HCl. MPM: Molecular weight Marker, Glu: Fraction eluted with glucose, Ara: Fraction eluted with arabinose, Gal: Fraction eluted with galactose, Lac: Fraction eluted with lactose, Mal: Fraction eluted with maltose, Man: fraction eluted with mannitol, Sac: Fraction eluted with saccharose, Sor: Fraction eluted with sorbitol, Fru: Fraction eluted with fructose. In the upper part sugar with which was performed the chromatographic elution and in the lower part number and molecular weight (kDa) of proteins present in each of the fractions.
Stem fractions extracted using the Tris-HCl method have a molecular weight ranging from 15 to 175 kDa (Figure 4), compared to NaCl method, this one presents a bigger number of bands.
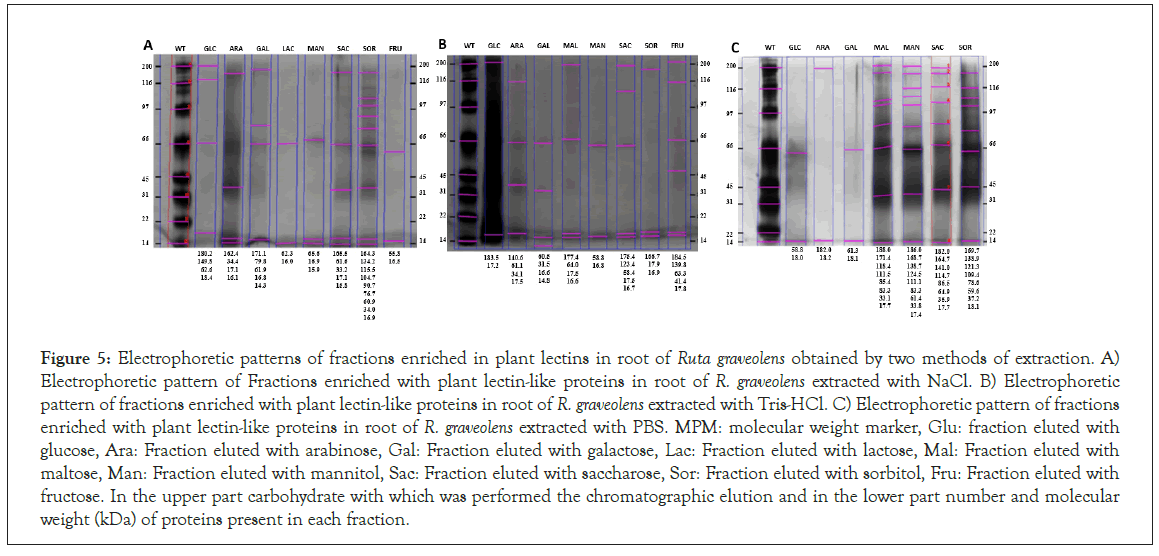
Figures 5 show the root extract electrophoretic profiles using the NaCl, Tris-HCl and PBS extraction methods, respectively.
Figure 5: Electrophoretic patterns of fractions enriched in plant lectins in root of Ruta graveolens obtained by two methods of extraction. A) Electrophoretic pattern of Fractions enriched with plant lectin-like proteins in root of R. graveolens extracted with NaCl. B) Electrophoretic pattern of fractions enriched with plant lectin-like proteins in root of R. graveolens extracted with Tris-HCl. C) Electrophoretic pattern of fractions enriched with plant lectin-like proteins in root of R. graveolens extracted with PBS. MPM: molecular weight marker, Glu: fraction eluted with glucose, Ara: Fraction eluted with arabinose, Gal: Fraction eluted with galactose, Lac: Fraction eluted with lactose, Mal: Fraction eluted with maltose, Man: Fraction eluted with mannitol, Sac: Fraction eluted with saccharose, Sor: Fraction eluted with sorbitol, Fru: Fraction eluted with fructose. In the upper part carbohydrate with which was performed the chromatographic elution and in the lower part number and molecular weight (kDa) of proteins present in each fraction.
Proteins, with molecular weight ranging from 14 to 188 kDa, are shown, having Tris-HCl and NaCl extraction methods a similar band pattern, whereas PBS extraction method gets a different band pattern.
Characterization of the hemagglutination effect of plant lectin-like proteins present in Ruta graveolens obtained by different extraction methods
The hemagglutination effect of lectin-like proteins was assessed in order to demonstrate the functional activity of those proteins through the demi purification process reported in the O+ blood type (Table 1).
| Extract | Fraction | Protein (mg) | Total activity (UHA) | Specific activity (UHA/mg) | Yield |
|---|---|---|---|---|---|
| % | |||||
| Leaf-NaCl | Crude | 1459.2 | 2 | 7.01 | 100 |
| Dialysate | 924.69 | 16 | 320 | 63.36 | |
| Enriched | 474.48 | 32 | 4195.5 | 32.51 | |
| Leaf-Tris/HCl | Crude | 4633.6 | 32 | 426.6 | 100 |
| Dialysate | 1721.6 | 8 | 80 | 37.15 | |
| Enriched | 625.41 | 8.8 | 382.5 | 13.49 | |
| Stem-NaCl | Crude | 1472.6 | 2 | 13.03 | 100 |
| Dialysate | 1135.4 | 4 | 53.3 | 77.1 | |
| Enriched | 667.3 | 22.2 | 1546.66 | 45.31 | |
| Stem-Tris/HCl | Crude | 1987.2 | 2 | 3.7 | 100 |
| Dialysate | 1180.3 | 16 | 640 | 59.39 | |
| Enriched | 596.7 | 20.44 | 1866.66 | 29.99 | |
| Root-NaCl | Crude | 737.3 | 16 | 640 | 100 |
| Dialysate | 395.3 | 16 | 640 | 53.61 | |
| Enriched | 359.4 | 24.22 | 2757.77 | 48.75 | |
| Root-Tris/HCl | Crude | 1821.8 | 32 | 2560 | 100 |
| Dialysate | 595.1 | 16 | 60 | 32.66 | |
| Enriched | 116.2 | 39.33 | 5264.44 | 6.38 | |
| Root-PBS | Crude | 358.4 | 32 | 2560 | 100 |
| Dialysate | 303.5 | 16 | 640 | 84.68 | |
| Enriched | 118.3 | 39.33 | 5264.44 | 33.01 |
Table 1: Hemagglutination activity of Ruta graveolens lectin-like proteins extracted from different organ by different extraction methods. UHA=the reciprocal of the highest dilution showing agglutination (2n). Specific Activity=hemaglutination units (UHA)/mg.
Hemagglutination activity of Ruta graveolens Lectin-like proteins extracted from different organ by different extraction methods. UHA=the reciprocal of the highest dilution showing agglutination (2n). Specific Activity=hemaglutination units (UHA)/mg.
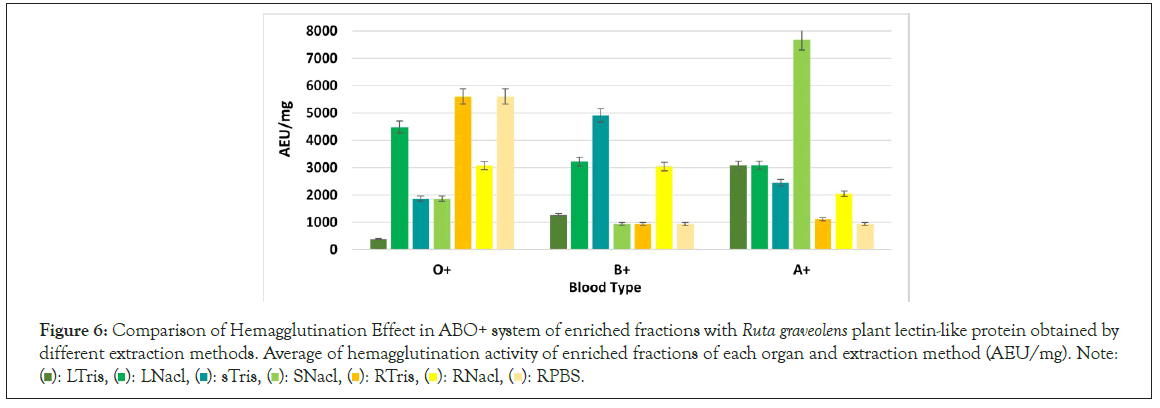
Most of R. graveolens Leaf and stem lectin-like proteins had hemagglutination effect in A+ blood type, whereas root lectins had hemagglutinating effect in the O+ blood type.
Lectin-like proteins had a global hemagglutination of 2560 AEU/mg, and a minimum concentration of 12.5 μg (Figure 6). Comparing the extraction methods, fractions extracted with NaCl showed a better biological effect in all organs, besides, the effect of the demipurification process relates to the increase of the biological activity through each enrichment process.
Figure 6: Comparison of Hemagglutination Effect in ABO+ system of enriched fractions with Ruta graveolens plant lectin-like protein obtained by different extraction methods. Average of hemagglutination activity of enriched fractions of each organ and extraction method (AEU/mg). Note: ( ): LTris, (
): LTris, ( ): LNacl, (
): LNacl, ( ): sTris, (
): sTris, ( ): SNacl, (
): SNacl, ( ): RTris, (
): RTris, ( ): RNacl, (
): RNacl, ( ): RPBS.
): RPBS.
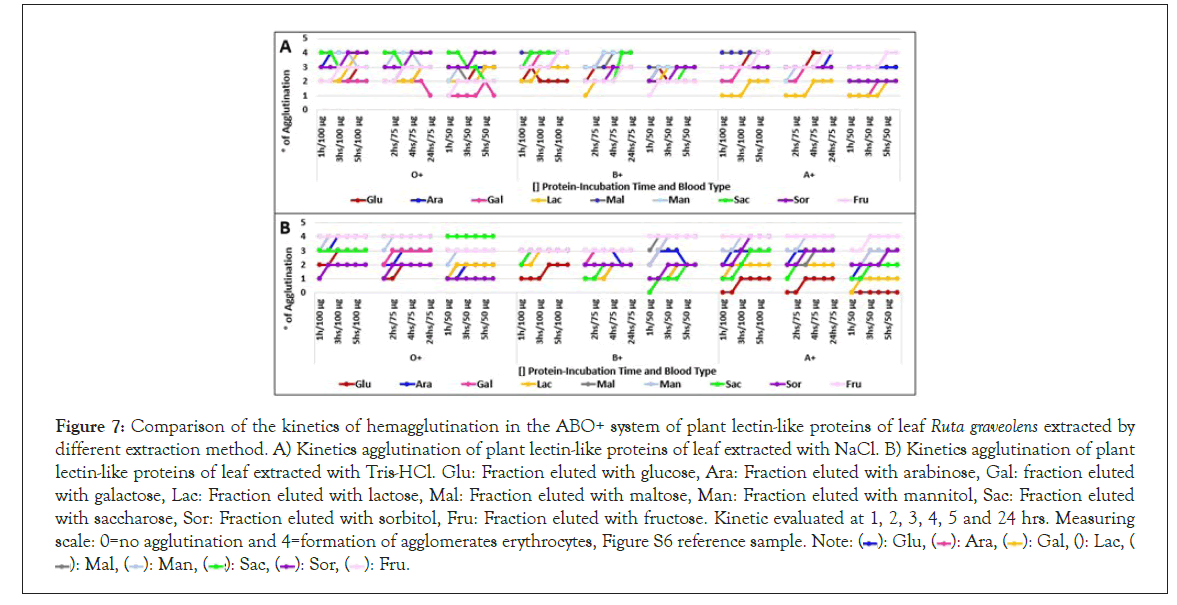
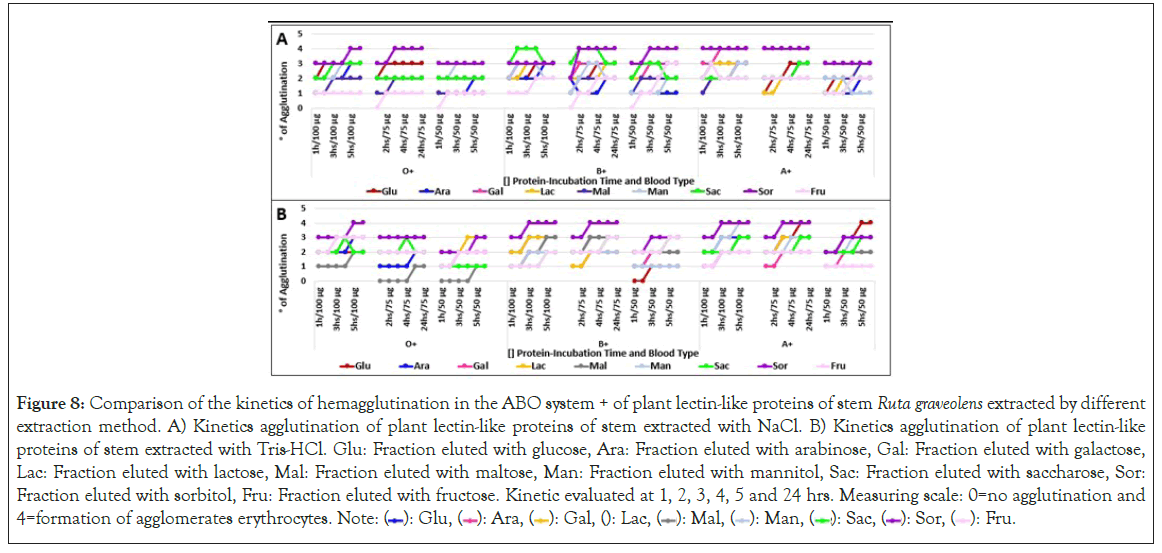
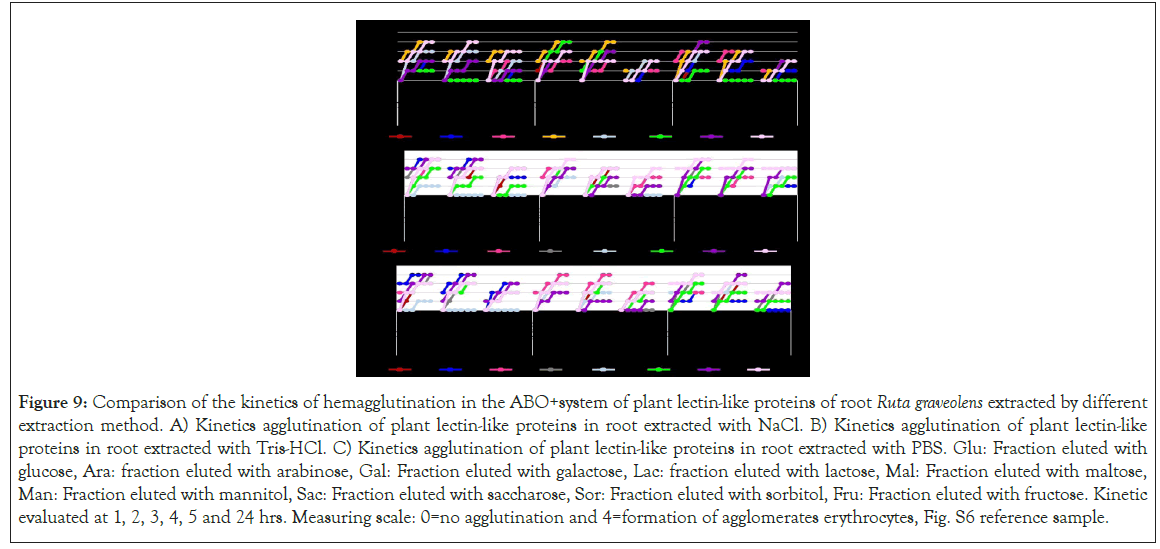
Hemagglutination kinetics was assessed identifying that most fractions reach their peak agglutination activity at 2 hours of incubation in high concentrations (100 μg/μl), being needed to extend its incubation time as protein concentration diminishes.
Not always high hemagglutination unit fractions had a high hemagglutination activity. Level 4 hemagglutination fractions were noticed at 1 hour of incubation, except root fractions, which required from 3 to 5 incubation hours to reach this hemagglutination level. Figures 7-9 suggesting that 2 hours are enough to saturate every carbohydrate binding site which interacts with the same sugars.
Figure 7: Comparison of the kinetics of hemagglutination in the ABO+ system of plant lectin-like proteins of leaf Ruta graveolens extracted by different extraction method. A) Kinetics agglutination of plant lectin-like proteins of leaf extracted with NaCl. B) Kinetics agglutination of plant lectin-like proteins of leaf extracted with Tris-HCl. Glu: Fraction eluted with glucose, Ara: Fraction eluted with arabinose, Gal: fraction eluted with galactose, Lac: Fraction eluted with lactose, Mal: Fraction eluted with maltose, Man: Fraction eluted with mannitol, Sac: Fraction eluted with saccharose, Sor: Fraction eluted with sorbitol, Fru: Fraction eluted with fructose. Kinetic evaluated at 1, 2, 3, 4, 5 and 24 hrs. Measuring scale: 0=no agglutination and 4=formation of agglomerates erythrocytes, Figure S6 reference sample. Note: ( ): Glu, (
): Glu, ( ): Ara, (
): Ara, ( ): Gal, (
): Gal, ( ): Lac, (
): Lac, ( ): Mal, (
): Mal, ( ): Man, (
): Man, ( ): Sac, (
): Sac, ( ): Sor, (
): Sor, ( ): Fru.
): Fru.
Figure 8: Comparison of the kinetics of hemagglutination in the ABO system + of plant lectin-like proteins of stem Ruta graveolens extracted by different extraction method. A) Kinetics agglutination of plant lectin-like proteins of stem extracted with NaCl. B) Kinetics agglutination of plant lectin-like proteins of stem extracted with Tris-HCl. Glu: Fraction eluted with glucose, Ara: Fraction eluted with arabinose, Gal: Fraction eluted with galactose, Lac: Fraction eluted with lactose, Mal: Fraction eluted with maltose, Man: Fraction eluted with mannitol, Sac: Fraction eluted with saccharose, Sor: Fraction eluted with sorbitol, Fru: Fraction eluted with fructose. Kinetic evaluated at 1, 2, 3, 4, 5 and 24 hrs. Measuring scale: 0=no agglutination and 4=formation of agglomerates erythrocytes. Note: ( ): Glu, (
): Glu, ( ): Ara, (
): Ara, ( ): Gal, (
): Gal, ( ): Lac, (
): Lac, ( ): Mal, (
): Mal, ( ): Man, (
): Man, ( ): Sac, (
): Sac, ( ): Sor, (
): Sor, ( ): Fru.
): Fru.
Figure 9: Comparison of the kinetics of hemagglutination in the ABO+system of plant lectin-like proteins of root Ruta graveolens extracted by different extraction method. A) Kinetics agglutination of plant lectin-like proteins in root extracted with NaCl. B) Kinetics agglutination of plant lectin-like proteins in root extracted with Tris-HCl. C) Kinetics agglutination of plant lectin-like proteins in root extracted with PBS. Glu: Fraction eluted with glucose, Ara: fraction eluted with arabinose, Gal: Fraction eluted with galactose, Lac: fraction eluted with lactose, Mal: Fraction eluted with maltose, Man: Fraction eluted with mannitol, Sac: Fraction eluted with saccharose, Sor: Fraction eluted with sorbitol, Fru: Fraction eluted with fructose. Kinetic evaluated at 1, 2, 3, 4, 5 and 24 hrs. Measuring scale: 0=no agglutination and 4=formation of agglomerates erythrocytes, Fig. S6 reference sample.
Discussion
Plant lectins are usually found in storage organs such as seeds [8,21] however, could also be found in leaves, stems, cortex and fruits, varying in concentration according to the plant organ evaluated [4,22].
There are very few reports referring to the extraction of plant lectins from vegetative tissues, most of them based on the use of saline solutions, some of them present variations on the pH, or consider the addition of other compounds, such as ascorbic acid, Triton X-100, among others [13,21,22].
Comparing the yield reported by Adenike and Babalola et al. [22] for Kalonche crenata leaves, Yan et al., [23] in 2009 for Astragalus mongholicus roots and Liu et al., [23] for Pinellia pedatisecda stems whom obtained a total protein yield of 4.8%, 1,8% y 3,5%, respectively, which is similar to the yield obtained in this study, even though the extraction method used was PBS buffer, having a medium ionic force., while Andrade et al., [24], for Bauhinia monandra leaves, and He et al., [25] for Ophioglossum pedunculosum roots obtained a 1.6% proteic yield, using a NaCl solution, confirming that saline solutions are an adequate method of total protein extraction, allowing to obtain plant lectins.
In this study two plant lectin extraction methods were assessed, obtaining through the NaCl extraction method a yield of total protein of 1.45% in leaves, 1.47% in stems and 0.737% in roots, while using Tris-HCl extraction method, the protein yield was 4.63% in leaves, 1.98% in stems, and 1.82% in roots.
According to these results, Tris-HCl extraction method allows to obtain a bigger protein concentration, confirming that this method is adequate to obtain a bigger amount of total protein.
After demi purification methods were performed, through dialysis and affinity chromatography, lectin-like proteins yield, comparing to total protein yield, was 32.5% in leaves extract, 45.3% in stem extract and 48.78% in root extract, through the NaCl extraction method, while using the Tris-HCl method, the lectin-like protein yield, comparing to total protein obtained was 13.49% in leaves extract, 30% in stem extracts and 6.58% in root extract; opposing to what Torres reported in 2006 [26], who stablished the Tris-HCl extraction method as the best to obtain lectin-like proteins, as our results supports that a bigger yield can be obtained through a high- ionic force solution, such as NaCl.
In the other hand, our results differ from the ones reported by Flores [2], where lectin-like protein obtained from Ruta graveolens yields through the NaCl extraction method were 3.34%, 2.55% and 1.51% out of leaves, stem, and roots, respectively. These results could be related to seasonal differences in different molecules yielding, as this study was performed near blooming season, while 2012 study was performed through January and February. There’s evidence sustaining seasonal changes in lectin production trough blooming and winter seasons [22,27,28].
A widely reported lectin-like protein demi purification technique was affinity chromatography, which exploits the high affinity to certain chemical groups establishing non-covalent bonds, and then being eluted using a free ligand [29,30].
The most commonly used matrixes for lectin-like protein purification are Sephadex, which is a glucose complex with free OH and CH2 groups, and Sepharose, which consists of two galactose molecules with free CH2 and OH groups, which could also have active thiol, oxirane and ester groups, allowing binding to C=O, N=N and C=C structures, interacting with OH, NH2 and SH groups, however, acrylamide and silica gel matrixes have also been reported in this context [31].
Silica gel has two oxygen molecules bound to silica trough double bounds, getting a similar structure to the one found in an ester group, allowing the protein and silica gel interactions and connections. Pereira et al., [13] reported the use of a silica gel matrix as a second demi purification step to obtain Colocasia esculenta lectins, while in this study a silica gel matrix was used in a simple chromatography, where lectin-like proteins were obtained.
Other studies establish glucose elution as the main method to separate lectin-like proteins from chromatography columns [5,7,32] however, the yield reported by Flores in 2012 allowed to obtain from 6 to 13% of lectin-like proteins from Ruta graveolens, while this study’s results establish a yield ranging from 32% to 49%, which can be attributed to the use of 9 different eluents in the affinity chromatography, allowing to consider the existence of a wider variety of lectin-like proteins, with affinity to different carbohydrates.
Total sugars, reducing and non-reducing sugars were quantified as part of the partial characterization of the different fractions, where data suggest that there is a bigger abundance of non-reducing sugars in the protein structure, adding up to 95% of all the sugars in fractions obtained from R. graveolens, with a protein-carbohydrate ratio of 0.1 to 5 mg, however, most carbohydrates associated with lectin-like protein structures reported are reducing sugars [18], which constitutes oligosaccharide elongation cores, while non- reducing sugars could be part of the peripheral region.
Furthermore, comparing both extraction methods, even though Tris-HCl extracted fractions had a bigger protein concentration, had a smaller protein-carbohydrate ratio, suggesting that the lower ionic-force method could allow obtaining different lectin- like proteins, against the possible obtainable lectin-like proteins through NaCl extraction methods. Another possible explanation could be the loss of the glycosidic bond when diminishing the carbohydrate concentration.
Lectin-carbohydrate interactions are assessed through the hemagglutination assay, process, getting two crossing types: (I) A bivalent lectin-like protein crossing with a monovalent carbohydrate in one dimension and (II) multivalent lectins and carbohydrates crossing, as one of the molecules has a bigger valence and creates two or three dimensions complexes that could precipitate when in solution, being the latter the crossing experienced in hemagglutination processes.
The bigger hemagglutination effect was assessed when fractions from leavers and stem interacted with A+ blood type, while root fractions had a bigger effect when interacting with root fractions. These results agree with the ones obtained by Hernández in 2011 [1] and Flores in 2012 [2], suggesting a higher affinity to N-acetyl galactosamine from leaf and stem lectin-like proteins, while root lectin-like proteins had a higher affinity to L-fucose.
Even though R. graveolens Lectin-like proteins seem to have higher affinity to certain blood type, hemagglutination activity in other blood type red cells was assessed, so it’s possible to classify them as non-specific lectin like proteins [22]. However, it’s important to consider that fractions were demi purified, so it’s possible that we could have an unspecific mix of lectin-like proteins, or the presence of more than one recognition domain.
It’s important to consider that temperature, pH, and metallic ion binding could affect the non-covalent interactions present in non-covalent interactions, leading to total or partial loss of lectin- like protein structure and biological activity [33,34]. Lectins are amphoteric and, as such, they can accept or donate protons, so pH changes affect their ionization state, changing its isoelectric point, resulting in Van Der Waals forces changes, promoting structure changes [29,30] thus, metallic bonds and their biological effect can be affected. This could explain the differences among fractions obtained from the same tissues, eluted with the same sugar but extracted with a different saline solution, being NaCl extracted fractions the ones with a higher AEU/mg range, comparing to the ones extracted with Tris-HCl buffer.
According to Hernández in 2011 [1] and Torres et al., the pH stability range of R. graveolens Lectin-like proteins is ranging from 5.4 to 8.4, even though these differences could be associated to pH differences in both solutions, both have a pH located in the stability range, suggesting different lectin-like proteins as the main possible reason why the hemagglutination effect is different. Loss of structural carbohydrates, ionization changes or metallic ion sequestering by Tris-HCl could be cited as possible structural change causes, thus changing the affinity and exposition of specific binding sites [20].
Comparing both extraction methods through hemagglutination kinetics, it was observed that similar protein concentrations led to similar agglutination levels, suggesting that this activity relates to the concentration needed to allow the agglutination to happen, while agglutination level relates to the number of molecules that could bind to a cell or lectins.
Furthermore, comparing among the same agglutination level simples, incubation time was higher for those extracted with low- ionic force solutions, such as Tris-HCl.
Conclusion
In Ruta graveolens extracts were found a variety of lectin-like proteins, showing affinity to different carbohydrates, especially at the roots and stems. It was demonstrated that a high-ionic force buffer allows obtaining a higher lectin-like protein yield, as well as a better performance at biological assessments.
These proteins have as part of their structure reducing carbohydrates, as part of their glycosylated chains cores, and non-reducing chains as part of their glycosylated chains residues, ranging from a 180 kDa.
The amount of protein bands present in the enriched fractions seems to be related to a higher protein concentration, as well as a better hemagglutination activity, which is evident through the high agglutination levels and shorter incubation time, on average being 3 hours enough to reach the higher agglutination level, with a minimal lectin-like protein concentration of 12,5 mg/ml, where leaf and stem lectin-like proteins had higher affinity to A+ blood- type red cells, while root lectin-like proteins had higher affinity to O+ blood-type red cells.
In this work we present preliminary characterization results of lectin-like proteins from R. graveolens, presenting the need to explore each protein identified in this plant, to better understand its ethnobotanic use. These results also allow us to propose alternatives to obtain lectin-like proteins, needing to complete the characterization and biological activity assessment.
Acknowledgement
We thank Dr. Espinosa Organista David Nahum, head office, and M.C. Genaro Montaño Arias, Unidad Multidisciplinaria de Investigación experimental Zaragoza UMIEZ, coordinator for their support at the “División de Estudios de Posgrado e Investigación”.
References
- Hernández J, Fenton B. Lectinas en Hojas de ruda (Ruta graveolens L). Purificación y Caracterización. Michoacan University of Saint Nicholas of Hidalgo. 2011.
- Hivrale AU, Ingale AG. Plant as a plenteous reserve of lectin. Plant Signal Behav. 2013;8(12):26595.
- Alvarez AT, GarcÃa LF, Mendoza-V E, Machuca C. Comparison between two extraction methods of plant lectins from Ruta graveolens L and their partial characterization. 2023.
- Vázquez-Luna A, Rivadeneyra-DomÃnguez E, DÃaz-Sobad R. Lectins in fruits and edible plants: New possibilities for interaction between food science and biomedicine. CienciaUAT. 2012;6(3):60-66.
- Hernández DÃaz P, MartÃn González O, RodrÃguez de Pablos Vélez Y, Ganem Báez FA. Applications of lectins. Rev Cuba de Hematol Inmunol. 1999;15(2):91-95.
- Jiang QL, Zhang S, Tian M, Zhang SY, Xie T, Chen DY, et al. Plant lectins, from ancient sugarâ?binding proteins to emerging antiâ?cancer drugs in apoptosis and autophagy. Cell Prolif. 2015;48(1):17-28.
- Mendoza W, Gandolfo L, Ponce L, Novello J, Marangoni S. Structure and function studies of a lectin isolated from Caesalpinia spinosa kuntze (tara) seeds. Idesia (Arica). 2007;25(2):49-58.
- Castillo-Villanueva A, Abdullaev F. Lectinas vegetales y sus efectos en el cáncer. Revista de investigación clÃnica. 2005;57(1):55-64.
- Ouyang L, Chen Y, Wang XY, Lu RF, Zhang SY, Tian M, et al. Polygonatum odoratum lectin induces apoptosis and autophagy via targeting EGFR-mediated Ras-Raf-MEK-ERK pathway in human MCF-7 breast cancer cells. Phytomedicine. 2014 15;21(12):1658-1665.
- Kabir SR, Zubair MA, Nurujjaman M, Haque MA, Hasan I, Islam MF, et al. Purification and characterization of a Ca2+-dependent novel lectin from Nymphaea nouchali tuber with antiproliferative activities. Biosci Rep. 2011;31(6):465-75.
- De Hoff PL, Brill LM, Hirsch AM. Plant lectins: The ties that bind in root symbiosis and plant defense. Mol Genet Genomics 2009;282:1-5.
- López I. Importance and use of lectins from Pleurotus sp. National Autonomous University of Mexico. 2013.
- Pereira PR, Winter HC, VerÃcimo MA, Meagher JL, Stuckey JA, Goldstein IJ, et al. Structural analysis and binding properties of isoforms of tarin, the GNA-related lectin from Colocasia esculenta. Biochim Biophys Acta. 2015;1854(1):20-30.
- Baldwin RL. How Hofmeister ion interactions affect protein stability. Biophys J. 1996;71(4):2056-2063.
- Flora vascular [Internet].
- Villarrubia o, Dubet m, Menéndez j, De la fuente j. Studies of 2 phytohemagglutinin preparations obtained by different methods. Rev Cienc Biol. 1995.
- Torres I. Purification and partial characterization of a lectin from tepary bean (Phaseolus acutifolius) with cytotoxic activity on cancer cells. Autonomous University of Querétaro. 2010.
- Noble JE, Bailey MJA. Quantitation of Protein. Methods in Enzymology. Elsevier. 2009;73-95 Walker JM. The Protein Protocols Handbook . Totowa. Humana Press. 2009.
- RodrÃguez MV, Riquelme B, Valverde J. Characterization of the binding effect of lectins from the Fabaceae family on human erythrocytes by image analysis. Anales AFA. 2004;16.
- Komatsu S, Yamada E, Furukawa K. Cold stress changes the concanavalin a-positive glycosylation pattern of proteins expressed in the basal parts of rice leaf sheaths. Amino Acids. 2009;36(1):115-123.
- Adenike K, Eretan OB. Purification and partial characterization of a lectin from the fresh leaves of kalanchoe crenata. Haw. BMB Reports. 2004;37(2):229-233.
- Yan Q, Li Y, Jiang Z, Sun Y, Zhu L, Ding Z. Antiproliferation and apoptosis of human tumor cell lines by a lectin of Astragalus mongholicus. Phytomedicine. 2009;16(7):586-593.
[Google Scholar] [PubMed]
- Liu X, Tian X, Liu T, Liang J. Disclosure of the tuberous lectin composed of homogeneous tetramers in pinellia pedatisecda schott. Appl Biochem Biotechnol. 2010;162(4):1214-1223.
[Crossref] [Google Scholar] [PubMed]
- Andrade CAS, Baszkin A, Santos-Magalhaes NS, Coelho LCBB, De Melo CP. Dielectric properties of Bauhinia monandra and Concanavalin A lectin monolayers, part I. J Colloid Interface Sci. 2005;289(2):371-378.
[Crossref] [Google Scholar] [PubMed]
- He X mei, Ji N, Xiang X cong, Luo P, Bao J ku. Purification, Characterization, and Molecular Cloning of a Novel Antifungal Lectin From the Roots of Ophioglossum pedunculosum. Appl Biochem Biotechnol. 2011;165(8):1458-1472.
[Crossref] [Google Scholar] [PubMed]
- Torres. Purification and partial characterization of a lectin from tepary bean (Phaseolus acutifolius) with cytotoxic activity on cancer cells. Autonomous University of Querétaro, Mexico. 2006.
- Berg J, Tymoczko J, Stryer L. Biochemistry Reverté Editorial, Sixth edition. 2008;70. [Crossref] [Google Scholar] [PubMed]
- Nelson D and Cox M. Lenhinger principles of biochemistry w.h. freeman and company, fifth edition. 2003;88.
- Valcarcel M. Analytical separation techniques. Editorial Reverté. Spain. 1998;335-336.
- Muñoz K, Londoño J, Arango G, Sierra J and Bravo K. Effect of the Ruta graveolens extraction technique on antityrosinase activity and correlation between enzymatic inhibition, phenolic compound content and cytotoxicity. Pharm Chem. J. 2007;2:78-83.
- Bernal C. Physicochemical and structural studies of the protein-protein and protein-ligand interaction of a lectin from the mussel Mytilus edulis. National Autonomous University of Mexico. Faculty of Higher Studies Cuautitlán, Mexico. 2007.
- Biswas S, Agrawal P, Saroha A, Das HR. Purification and mass spectrometric characterization of sesbania aculeata (dhaincha) stem lectin. Protein J. 2009;28(10):391-399.
[PubMed]
- Gabius HJ, André S, Jiménez-Barbero J, Romero A, SolÃs D. From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem Sci. 2011;36(6):298-313.
[Crossref] [Google Scholar] [PubMed]
- Torres M. Effect of Ruta graveolens L Stem lectins in a murine model of cáncer. National Autonomous University of Mexico. Faculty of Higher Studies Zaragoza. 2015.
Citation: Torres AA, Mendoza-VE, Flores GL, Machuca RC. (2024) Comparison between two Extraction Methods of Plant Lectins from Ruta graveolens L. and their Partial Characterization. Bio Med. 16:636.
Copyright: © 2024 Torres AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.