Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 0, Issue 0
Comparative Investigation of Commercially Available Anti-Bacterial Creams for their Efficacy Against Different Bacterial Strains
Parthika Patel*, Isha Patel, Sayli Kachare and Priyanka MauryaReceived: 19-Jul-2024, Manuscript No. JBP-24-26545 ; Editor assigned: 22-Jul-2024, Pre QC No. JBP-24-26545(PQ); Reviewed: 05-Aug-2024, QC No. JBP-24-26545 ; Revised: 13-Aug-2024, Manuscript No. JBP-24-26545 (R); Published: 21-Aug-2024, DOI: 10.35248/2155-9597.24.S27.100
Abstract
Introduction: Antibacterial creams play a vital role in the treatment of bacterial skin infections. With numerous products available, these creams differ significantly in concentration, price, activity levels, active ingredients and excipients. The choice of cream is critical for effective treatment and depends on the specific bacterial strains involved. Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Bacillus subtilis (B. subtilis) and Proteus vulgaris (P. vulgaris) are four bacterial strains commonly associated with human illnesses and skin disorders. This study aims to evaluate the antibacterial efficacy and economic viability of four popular commercial antibacterial creams containing either Gentamicin sulfate, Framycetin sulfate, or a combination of Silver sulfadiazine, Miconazole nitrate, Neomycin sulfate and Chlorhexidine gluconate. The objective is to identify the most effective and economical option among these creams.
Materials and Methods: This study was designed to evaluate the antibacterial efficacy and economic viability of four popular commercial antibacterial creams against four bacterial strains commonly responsible for human skin infections. The study employed a laboratory based experimental design using the disk diffusion method to assess the antibacterial activity of each cream.
Results and Conclusion: Gentamicin sulfate emerges as the top-performing antibacterial cream in this study, exhibiting the widest zones of inhibition against key bacterial strains. Its potent antibacterial efficacy is complemented by favorable physical properties such as low pH, high spreadability and low viscosity. These findings position Gentamicin sulfate as a preferred choice for effectively treating bacterial skin infections.
Keywords
Antibacterial creams efficacy; Disk diffusion method; Staphylococcus aureus inhibition; Comparative antibacterial study; Topical antibiotic effectiveness
Abbrevations
S. aureus: Staphylococcus aureus; B. subtilis: Bacillus subtilis; E. coli: Escherichia coli; P. vulgaris: Proteus vulgaris; G+ve: Gram Postive; G-ve: Gram negative; MRSA: Methicillin resistant S. aureus; PMF: Proteus vulgaris fimbriae; GIT: Gastrointestinal Tract; UTI: Urinary Tract Infections; ETEC: Enterotoxigenic E. coli; EHEC: Enterohaemorrhagic E. coli; UPEC: Uropathogenic E. coli; HUS: Hemolytic Uremic Syndrome; IP: Indian Pharmacopoeia; ZOI: Zone of Inhibition
Introduction
‘Comparative’ comes from the verb ‘to compare’. The name comes from the Latin ‘comparare’, which is connected to the phrase par and ‘equal’, as well as the letter com. A careful analogy can be draw from it [1].
Comparison studies examine similarities and/or differences between numerous, but separate, locations, entities and/or themes using both qualitative and quantitative comparison methodologies for event analysis and evaluation.
Comparative technique is the act of comparing two or more entities and/or things that are either similar to one another or dissimilar from one another. Statistics that show consistent or inconsistent relationships between two or more entities are found through comparative investigations between variables.
Introduction to anti-bacterial creams
Dermal medicines are ideal for localized drug delivery since they distribute medications directly to the site of action. In the tissue where the action is being carried out, this strategy allows for great antibacterial bioavailability. Further benefits of topical antibacterial therapy include low drug concentrations, low cost and no alteration in the body's normal gut flora. The amount that antibacterial lotions penetrate a person's epidermis depends on their skin type, the concentration of the active component and the size of the molecules. Topical treatments usually aim to target the skin's uppermost layer, known as the epidermis. A sufficiently soluble molecule can permeate this layer, the dermal layer that follows, numerous layers of the dermis and the subcutaneous layer, which is the lowest layer of the skin (Tables 1-4) [2].
| Sr no. | Cream details | Description |
|---|---|---|
| 1 | Drug name | Framycetin sulphate |
| 2 | Brand name | Soframycin |
| 3 | Manufactured by | Encube Ethicals Pvt. Ltd. |
| 4 | Marketed by | Encube Ethicals Pvt. Ltd. |
| 5 | Composition | 1% w/w Framycetin sulphate IP |
| 6 | Content of cream | 30.0 grams |
| 7 | Category | Bactericidal cream |
| 8 | Utilization | It cures bacterial infections of the skin, hair, nails and external ears. |
| 9 | Cost | Rs. 55 |
Table 1: General description of marketed anti-bacterial creams used for the study framycetin sulphate cream.
| Sr no. | Cream details | Description |
|---|---|---|
| 1 | Drug name | Gentamicin sulphate |
| 2 | Brand name | Gentlee |
| 3 | Manufactured by | Suyaash pharmaceuticals |
| 4 | Marketed by | Atopic laboratories Pvt.Ltd. |
| 5 | Composition | 0.3% Gentamicin sulphate IP |
| 6 | Content of cream | 20.0 grams |
| 7 | Category | Anti-bacterial cream |
| 8 | Utilization | Bacterial skin infections, minor cuts, scrapes burns, dermatitis and eczema. |
| 9 | Cost | Rs. 20 |
Table 2: General description of Gentamicin Sulphate cream.
| Sr no. | Cream details | Description |
|---|---|---|
| 1 | Drug name | Clobetasol propionate, neomycin sulphate, miconazole nitrate |
| 2 | Brand name | Clobenate-GM |
| 3 | Manufactured by | Prochem pharmaceuticals Ltd |
| 4 | Marketed by | Swifr |
| 5 | Composition | Clobetasol propionate (0.05%), neomycin sulphate (0.5%), miconazole nitrate (2.0%) |
| 6 | Content of cream | 10.0 grams |
| 7 | Category | Antibacterial cream |
| 8 | Utilization | Contact skin inflammation, dermatitis, psoriasis, eczema |
| 9 | Cost | Rs. 70 |
Table 3: General description of globetasol propionate, neomycin sulphate and miconazole nitrate cream.
| Sr No. | Cream details | Description |
|---|---|---|
| 1 | Drug name | Silver sulphadiazine and chlorhexidine gluconate |
| 2 | Brand name | Silvorest |
| 3 | Manufactured by | Novanta Healthcare LLP |
| 4 | Marketed by | Sunrest lifescience Ltd |
| 5 | Composition | Silver sulphadiazine (1.00%) IP chlorhexidine gluconate (0.20%) |
| 6 | Content of cream | 15.0 grams |
| 7 | Category | Anti-bacterial cream |
| 8 | Utilization | second and third-degree burn. |
| 9 | Cost | Rs. 60 |
Table 4: General description of silver sulphadiazine and chlorhexidine gluconate cream.
Most of them fall under one of two main groups of antibacterial:
Antiseptics: These are cleaning agents that can be used to get rid of or stop some open wounds and bacteria that might stick to the skin.
Antibiotics: These chemicals are produced by bacteria and possess the authority for either destroy or hinder advancement of other microbes.
This study includes the use of following four antibacterial creams:
• Framycetin Sulphate (single drug cream)
• Gentamicin Sulphate (single drug cream)
• Clobetasol Propionate, Neomycin Sulphate and Miconazole Nitrate (Combination of three drug’s cream)
• Silver Sulfadiazine and Chlorhexidine Gluconate (Combination of two drug’s cream)
Materials and Methods
Broad overview of various bacteria which was used
Microbes with a single cell that breathe continuously and divide via binary fission are called bacteria. They do not have a nucleus. Based on scientific studies, they are a major contributor to illness. Bacteria are extremely sophisticated and diverse animals, despite their seemingly simple appearance. A vast array of hydrocarbon substrates, including phenol, rubber and petroleum, can be fed to bacteria of various sorts, which can proliferate quickly. These animals can live as autonomous or parasitic entities and they are widely dispersed. All areas of medicine involve bacteria and it is important to recognize that they may adapt to constantly changing surroundings by selecting for spontaneous mutations.
• S. aureus
• P. vulgaris
• B. subtilis
• E. coli
S. aureus
S. aureus is a naturally occurring bacteria that lives in the mucous membranes and skin of humans and animals. Furthermore, oxygen is not necessary for it to function because it is facultatively anaerobic. Humans are susceptible to a wide range of diseases, from common Dermatitis and soft tissue disorders that could possibly cause fatal conditions such as heart disease, septicemia caused by S. aureus infections [3-11].
What distinguishes it most is its ability to withstand osmotic and ionic stress. Because of its high tolerance, this sickness, for example, can persist in highly salinized environments. The bacteria’s resistance to the above-mentioned stimuli depends on their capacity to produce and osmotically absorb large quantities of internal Osmo protectants from external sources. Pathogenesis requires most of the exotoxins produced by S. aureus. These particular protein types enable bacteria to stick on the surfaces of cells, get past the host's defences and eventually cause cell death. Super-antigens are one of them that can cause toxic shock syndrome. The cytolysin beta-toxin is one of the numerous that damage the membranes of eukaryotic cells.
Pathogenic effect: Antimicrobial peptides, antibacterial weaponry and virulence factors enable S. aureus to outsmart its competitors and evade the immune system. It is also associated with staph and releases several cytolytic toxins, such as PVL, beta and alpha-toxins and others. Along with other exoenzymes that damage host tissues and promote bacterial dispersion, S. aureus also produces proteases, coagulase, hyaluronidase and others.
S. aureus has also developed a number of incredibly complex defensive mechanisms to withstand the effects of a broad range of antimicrobial medications. These processes include the creation of bio-film and beta-lactamases, which confer resistance to beta-lactam antibiotics such as penicillin and methicillin. Bacteria can take on new shapes on both biotic and abiotic surfaces. Through a process known as bio-film formation, which creates a safe growth environment on both biotic and abiotic tissue, bacteria can tolerate host reactions from the immune system.
Disease caused: Due to the presence of commensal S. aureus bacteria, this is in extremely fine condition. In the event that the host's immunity is compromised or the skin barrier is broken, illnesses may result. Folliculitis and impetigo are two of the most common soft tissue diseases caused by S. aureus. The primary cause of these diseases is bacteria, which can attach themselves to host tissues and release toxins capable of causing tissue destruction. Osteomyelitis, pneumonia, endocarditis and bacteremia are a few potentially lethal invasive illnesses linked to substantial S. aureus infections. Because of its high transmissibility and resistance to multiple treatments, MRSA poses serious therapeutic problems that call for extreme vigilance.
P. vulgaris
P. vulgaris is one of the most renowned species of the Proteus genus because it is known to infect people. By analysing the larvae of the gipsy moths, or Porteria dispar, Proteus myxofaciens was detected. It represents the lone Proteus species that doesn't appear to have any impact upon human toxicity. On the other hand, P. vulgaris is often associated with respiratory and urinary tract infections, as well as excrement and wound samples. P. vulgaris can be grown on a variety of media types, including 6% blood agar, MacConkey agar and Teepol lactose agar. Swarm expansion is a feature of P. vulgaris when the organism spreads out as a single film across the surface of the plate. P. vulgaris proliferates characteristically when its ‘swimming cells’ in broth transform into ‘swarmer cells’ on the surface of agar. Only after enhanced flagellin synthesis and cellular elongation does this happen [12-16].
P. vulgaris is frequently linked to urinary tract infections of the complex variety. Acute pyelonephritis, cystitis, kidney or bladder stones, urolithiasis and even bacteremia can all result from it, which often affects the upper urinary tract. P. vulgaris can also, in rare instances, cause wound infections and meningitis in infants or young children.
To combat the illness caused by this protein-causing bacterium, one must comprehend the main virulence factors of P. vulgaris. PMF and cell adherence to the uroepithelium are the two main factors associated with virulence. Significant effects of the factors include kidney, bladder and urinary tract stone development and catheter colonization.
The microbiological opportunist parasite P. vulgaris necessitates treatment and ongoing monitoring, particularly in cases of complicated UTIs and other co-occurring conditions. In order to develop effective treatment strategies for infections caused by P. vulgaris, further research is needed to gain a deeper understanding of the pathogen's virulence characteristics.
Pathogenic effect: To help it carve out and hold onto its environmental niche in the face of rival bacteria, P. vulgaris possesses an incredible array of defense mechanisms. Proteolytic enzymes, including various proteases, are essential components of its toolkit since they aid in the degradation of host proteins and extracellular matrix components. Proteases not only nourish the organism but also facilitate tissue invasion and the breakdown of host defenses by breaking down peptide links in proteins.
Vulgaris expresses flagella and pili, motility-related features that allow it to travel across surfaces and penetrate host tissues. Because of its increased mobility, it can move around more readily and evade the immune system's identification. By creating a permanent safe haven and utilizing motility therapies, it is able to form bio-films on both biotic and abiotic surfaces, increasing its resistance to antimicrobial drugs.
Disease caused: People with weakened immune systems or any other medical condition that puts them at risk are frequently exposed to P. vulgaris, which can lead to opportunistic infections. It is thought to be a common bacterium found in the human gastrointestinal tract. The infection-causing bacterium often manifests as a single kind of UTI. This particular kind of UTI is identified more precisely as microorganisms that produce urease and colonise the urinary system in an ascending fashion, eventually resulting in the creation of bladder crystals, or stones, known as ‘struvite stones’. P. vulgaris has been associated with sepsis, abscess’s and wound infections, in addition to urinary tract infections. Meningitis and endocarditis are examples of potential side effects. Drug-resistant strains of P. vulgaris are a major concern, of course, as they provide a barrier to therapeutic therapy and necessitate the development of novel strategies for infection control.
B. subtilis
B. subtilis is an electrically charged bacteria that is G+ve in the Gram stain. Among other places, it can be found in soil, water and the sky. It comes in comprehensive form. Spores of the species adapt to environments that challenge the bacteria's ability to flourish. For numerous studies on its biochemistry and genetics, B. subtilis has served as a model organism. Biotechnology, industry and agriculture all employ B. subtilis extensively; however, for those with compromised immune systems, handling the bacteria might result in respiratory tract infections or ulcers [17-26].
Foods contaminated with B. subtilis have also been linked to food-borne illness. Remarkably, B. subtilis is not as dangerous to humans as other bacteria. To sum up, there are ways to greatly reduce or eliminate the hazards connected to its exposure. Maintaining the highest standards of personal hygiene, washing and sanitizing hands often and adhering to safety protocols can all help achieve this.
Pathogenic effect: Surfactin, Bacillomycin and subtilin are only a few of the antibacterial compounds that B. subtilis produces and secretes to show its antibacterial action. B. subtilis uses a variety of methods to manufacture a spectrum of antibiotic substances, including Subtilin, Bacillomycin and Surfactin. In addition, the company produces a range of materials that are effective against pathogens, such as MRSA and other bacteria. Most bacterial diseases can be effectively treated and cured with these medications. One such material is Surfactin, which functions by breaking the lipid bilayer structure the antibiotic has formed, harming the bacterial cell wall, injuring the bacterial plasma membrane and so on. Bacillomycin and Subtilin also inhibit this pathway. Bacillomycin and Subtilin have shown the ability to impede bacterial growth by selectively targeting vital cell components. Potential diseases are indirectly prevented by encouraging competition among the population's microorganisms for resources and housing. Antibacterial structures that keep hazardous germs from settling become more effective agents, enhancing the microorganisms' potent ability to create bio films. It facilitates quorum-based communication, which improves the group's capacity to react collectively to threats posed by hazardous bacteria and reduces the amount of damage that these microbes may cause.
Disease causes: The beneficial and benign bacterium B. subtilis is often believed to have the potential to turn into an opportunistic pathogen in weak or sterile settings. The rare nature of these infections is described in medical literature. They arise in hospitals via tainted surgical implants and compromised medical equipment. These infections can result in septicemia, bacteremia, device-related infections and other dangerous side effects. When B. subtilis mutant strains are used in agriculture, there's a chance that they could unintentionally affect nearby microbial communities or host organisms, which could cause illnesses in plants or animals. Determining the origins of harmful strains of B. subtilis is necessary to reduce hazards and maintain public safety. This bacterium has to be safely discharged into the environment because it has so many applications. Its potent biofilm forming ability boosts its effectiveness against bacteria by both colonizing the surfaces that they reside on and erecting strong barriers against them. Furthermore, the organism defends against the possibility that other bacteria would suppress or replace them thanks to the signaling systems' capacity to compute population density.
E. coli
E. coli may produce a deadly infection in people, or it may be beneficial. It's the most commonly employed object in cloning research because of its easy genetic coding and fast replication. On the other hand, rare cases may occur where specific E. coli strain acquire virulence traits that make them extremely deadly to a variety of human illnesses [27-36].
Distinct E. coli patho types that infect humans are linked to different virulence factors and medical consequences. EPEC, EHEC and ETEC are the patho types of E. coli that are frequently observed. These patho types can result in meningitis or septicemia, cavities in the mouth and diarrheal or enteral diseases. In particular, ETEC produces enterotoxins that cause watery diarrhea. Hemolytic uremic syndrome and hemorrhagic colitis is thought to be associated with EHEC, EPEC and neonatal diarrhea.
Its primary distinguishing characteristics are its sticky consistency, look and toxins that harm cells and activate the immune system. The primary mechanism by which pathogenic E. coli strains cause illness and colonization is their ability to penetrate and adhere to host cells. Hemorrhagic colitis and hemolytic uremic syndrome may be made worse by EHEC strains that generate shiga toxin.
A multifunctional microorganism with potential benefits and drawbacks is E. coli. Because they produce virulence factors that harm host cells and suppress the immune system in host cells, pathogenic forms of E. coli could lead to a broad-spectrum human disease. Understanding how E. coli’s pathogenic strains enter our body, spread and because disease is essential for both infection prevention and treatment.
Pathogenic effect: Due to its capacity to outcompete other microbes, E. coli has a particular role in the gut microbiota. Its primary defense mechanism is the production of bacteriocins, which are minuscule antimicrobial peptides that specifically kill related types of bacteria. A variety of outer membrane proteins secreted by E. Coli regulate the flow as well. To help with population control and environmental adaptation, E. Coli employs a method known as quorum sensing. It is feasible for group behavior and virulence elements to emerge in a bio-film at the same time. Additionally, the ability of these bacterial cells to take up transposons and plasmids with antibiotic-resistant determinant genes promotes the spread of resistance genes and horizontal gene transfer.
Disease cause: In most cases, EHEC bacteria produce the Shiga toxins that can lead to HUS, a potentially fatal infection. Bloody diarrhea can also be brought on by these poisons. But they adhere to intestinal walls and produce adherence and effacement lesions, which are often associated with E. coli infections (Steinberg and Levin 1994). Moreover, UPEC strains produce toxins in addition to adheresins, which help the bacteria attach to bladder walls and cause infections of the urinary tract as well as other ailments like pyelonephritis [28-36]
Preparation of nutrient agar medium and nutrient broth
An agar nutritional culture medium was made with agar, sodium chloride, peptone, distilled water and beef extract. The medium was heated to a complete dissolve in all of the ingredients. The medium was autoclaved for fifteen minutes in an autoclave set to 121°C and 15 Pascal pressure.
The nutritional agar medium is added in an aseptic state after each petri plate and nutritive agar medium have been sterilized. In order to give the agar time to solidify completely, the plates are cooled to room temperature and then put in the refrigerator, where they are kept for another day. For the purpose of making the nutrient-dense broth, beef extract, distilled water and sodium chloride were combined and brought to a boil. After that, the medium was infected for fifteen minutes at 121°C in an autoclave with a pressure setting of 15 Pascal. Proceed to fill four test tubes with the soup, up to two milliliters.
Bacterial strains
One colony of bacteria was introduced to the nutrient broth in order to form the bacterial suspension. This was done to ensure that a small number of cells were transferred to the colony when they came into contact with a sterile inoculating loop. The nourishing soup with the inoculating loop was shaken to allow the bacteria to multiply. The test tubes were then placed in an incubator with a temperature setting of 35°C-37°C to encourage the growth of bacteria.
Preparation of inoculum
By adding a single colony of bacteria to the nutritional broth, the bacterial suspension was produced. Making sure that a minimal amount of cells was added to the colony was the aim of this step. The contaminating loop was touched in order to transfer the cells. A gentle shake was given to the nutrient-rich broth after the inoculating loop was inserted into the test tube. In an incubator with a temperate climate (35°C-37°C), the test tubes were left to cultivate bacteria.
Preparation of a test solution for marketed creams
Four cream test solutions were prepared in accordance with IP. One mg of framycetin sulphate was present in each ml of pH 8 phosphate buffer in the framycetin sulphate cream test solution. As a result, the test solutions for gentamicin sulfate cream and neomycin sulfate cream contained 1 mg of gentamicin sulfate and 1 mg of neomycin sulfate, respectively, in 1 ml of pH 8 phosphate buffer. One milligram of silver sulfadiazine per milliliter of sterile water made up the silver sulfadiazine test solution [36-45].
Anti-bacterial activity of creams
Each cream's antibacterial effectiveness is evaluated using the disk diffusion method. Using a glass spreader, the particular bacterial culture medium was added to the nutrient agar plates and five wells were punched out with a borer. The four wells that encircled the central well were labeled with the particular test sample solution and one well was designated as control. As shown in the figure below, test solutions of the creams were pipetted into the appropriate wells. The wells were then incubated at 35°C-37°C for the entire day to allow the bacteria to grow. Each cream's antibacterial effectiveness is evaluated using the disk diffusion method. Using a glass spreader, the particular bacterial culture medium was added to the nutrient agar plates and five wells were punched out with a borer. The four wells that encircled the central well were labeled with the particular test sample solution and one well was designated as control. As shown in the figure below, test solutions of the creams were pipetted into the appropriate wells. The wells were then incubated at 35°C-37°C for the entire day to allow the bacteria to grow [46-50].
Results and Discussion
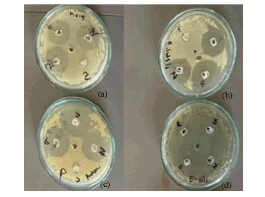
A zone of inhibition is a clearly demarcated, circular region surrounding antibiotic disks, wherein bacterial growth is precluded. A zone of inhibition can be measured in millimeters; as shown in Figure 1 and Table 5, following a 24-hour period of agar plate incubation.
Figure 1: ZOI of different anti-bacterial creams. Note: (a) P. vulgaris; (b) B. subtilis; (c) S. aureus (d) E. coli.
| Sr No. | Cream name | ZOI in different Bacterial strain (millimeter) | |||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. vulgaris | ||
| 1 | Framycetin sulphate | 2.8 mm | 2.9 mm | 2.0 mm | 3.1 mm |
| 2 | Gentamicin sulphate | 3.0 mm | 3.3 mm | 2.3 mm | 3.2 mm |
| 3 | Clobetasol propionate, neomycin sulphate & miconazole nitrate |
2.6 mm | 2.8 mm | 1.6 cm | 3.0 mm |
| 4 | Silver sulfadiazine & chlorhexidine gluconate |
1.2 mm | 1.0 mm | 1.1 mm | 1.3 mm |
Table 5: Observation table of ZOI of different anti-bacterial creams.
Using the disc diffusion method, the antibacterial activity of four antibacterial creams that were chosen from the market was assessed. Additionally, four distinct bacterial strains were employed, including G+ve strains of B. subtilis, S. aureus, E. coli and P. vulgaris. Table 5 and Figure 1 display the zones of inhibition of various creams on various bacterial strains; as can be seen, gentamicin sulfate has the largest zone of inhibition of all the creams in all bacterial strains.
The result shows that the cream containing gentamicin sulfate has the highest potential antibacterial activity among all the creams and bacterial strains. A single drug cream with strong antibacterial qualities that is reasonably priced is gentamicin sulfate. Based on our findings, there is little doubt that gentamicin sulfate cream is more effective than the other four creams at stopping the growth of bacteria. In both gram-positive and gram-negative bacteria, gentamicin sulfate cream exhibits the highest zone of inhibition. In addition to being cost effective-a critical component in the present world-the cream has the ability to battle bacteria effectively.
Conclusion
Using the antibacterial study described above as a guide, we identified the cream that was the most effective or showed the highest amount of antibacterial activity. The results also showed that gentamicin sulfate, as a single-ingredient drug, had the most potential when compared to combination creams, all at a low cost. In addition to patient compliance, contentment, safety, effectiveness and availability, this research helps medical experts determine which infections are best treated with particular creams.
Acknowledgement
The writers are grateful to Miss Parthika Patel of the SSR College of Pharmacy for her assistance and direction in this effort.
References
- Coccia M, Benati I. Comparative evaluation systems. Global Encyclopedia of Public Administration, Public Policy and Governance. 2018.
- Bandyopadhyay D.Topical antibacterials in dermatology. Indian J Dermatol. 2021; 66(2):117-125.
[crossref] [Google scholar] [Pubmed]
- Foster TJ Immune evasion by staphylococci. Nat Rev Microbiol.2005; 3(12):948-58.
- Foster TJ. Staphylococcus aureus. J Med Microbiol .2002; 1:839-888.
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998; 339(8):520-532.
[Crossref] [Google scholar] [Pubmed]
- Otto M. Staphylococcus aureus toxins. Curr Opin Microbiol. 2013;17:32-37.
[Crossref] [Google scholar] [Pubmed]
- Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009; 7(9):629-641.
[Crossref] [Google scholar] [Pubmed]
- Smith JA, Willis AT, O'connor JJ. New epidemic strains of Staphylococcus aureus. Lancet. 1965; 2(7416):772-3.
[Crossref] [Google scholar] [Pubmed]
- Bhakdi S, Tranum JJ. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991; 55(4):733-751.
[Crossref] [Google scholar] [Pubmed]
- Lyon BR, Skurray RO. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev.1987: 51(1):88-134.
- Easmon CS, Adlam C. Staphylococci and Staphylococcal Infections: The organism in vivo and in vitro. Academic press; 1983.
- Mohammed GJ, Kadhim MJ, Hameed IH. Proteus species: Characterization and herbal antibacterial: A review Int J Pharmacogn Phytochem Res. 2026; 8(11):1844-1854.
- Armbruster CE, Mobley HL. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol. 2012; 10(11):743-754.
[Crossref] [Google scholar] [Pubmed]
- Mobley HL, Belas R. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends in Microbiol.1995; 3(7):280-284.
[Crossref] [Google scholar] [Pubmed]
- Krajewska B. Ureases I Functional, catalytic and kinetic properties: A review. J Mol catal B Enzyme. 2019; 59(1-3):9-21.
- Bonadio M, Meini M, Spitaleri P, Gigli C. Current microbiological and clinical aspects of urinary tract infections. Eur urol. 2001;40(4):439-445.
[Google scholar] [Pubmed]
- Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol; 2005.56(4):845-857
[Crossref] [Google scholar] [Pubmed]
- Eijlander RT, Kuipers OP. Live-cell imaging tool optimization to study gene expression levels and dynamics in single cells of Bacillus cereus. App Env Microbiol. 2013;79(18):5643-5651.
[Crossref] [Google scholar] [Pubmed]
- López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010; 2(7):a000398.
[Crossref] [Google schoar] [Pubmed]
- Senesi S, Celandroni F, Tavanti A, Ghelardi E. Molecular characterization and identification of Bacillus clausii strains marketed for use in oral bacteriotherapy. Appl Environ Microbiol. 2001;67(2):834-9.
[Crossref] [Google scholar] [Pubmed]
- Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev.2010; 23(2):382-398.
[Crossref] [Google scholar] [Pubmed]
- Stenfors ALP, Fagerlund A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Reviews. 2008; 32(4):579-606.
[Crossref] [Google scholar] [Pubmed]
- Mols M, Abee T. Bacillus cereus responses to acid stress. Environ Microbiol. 2011; 13(11):2835-2843.
[Crossref] [Google scholar] [Pubmed]
- Lindbäck T, Mols M, Basset C, Granum PE, Kuipers OP, Kovács ÁT. CodY, a pleiotropic regulator, influences multicellular behaviour and efficient production of virulence factors in Bacillus cereus. Environ Microb. 2012;14(8):2233-46.
[Crossref] [Google scholar] [Pubmed]
- Wijman JG, Leeuw PP, Moezelaar R, Zwietering MH, Abee T. Air-liquid interface biofilms of Bacillus cereus: formation, sporulation and dispersion. Appl Environ Microbiol. 2007;73(5):1481-8.
[Crossref] [Google scholar] [Pubmed]
- Ramachandran R, Chalasani AG, Lal R, Roy U. A Broadâ?Spectrum Antimicrobial Activity of Bacillus subtilis RLID 12.1. Scintific World Journal. 2014; (1):968487.
[Crossref] [Google scholar] [Pubmed]
- Clermont O, Christenson JK, Denamur E, Gordon DM. The C lermont Escherichia coli phyloâ?typing method revisited: improvement of specificity and detection of new phyloâ?groups. Environ Microbiol Rep. 2013; 5(1):58-65.
[Crossref] [Gooogle schloar] [Pubmed]
- Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004; 2(2):123-140.
[Crosssref] [Google scholar] [Pubmed]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol biol Rev.2003; 67(4):593-656.
[Crossref] [Google scholar] [Pubmed]
- Russo TA, Johnson JR. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000 ;181(5):1753-4.
[Crossref] [Google scholar] [Pubmed]
- Nataro JP, Kaper JB. Diarrheagenic escherichia coli. Clin Microbiol Rev.1998;11(1):142-201.
[Crossref] [Google scholar] [Pubmed]
- Cassels FJ, Wolf MK. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J Ind Microbiol.1995;15(3):214-26.
[Crossref] [Google scholar] [Pubmed]
- Keller R, Ordoñez JG, de Oliveira RR, Trabulsi LR, Baldwin TJ, Knutton S. Afa, a diffuse adherence fibrillar adhesin associated with enteropathogenic Escherichia coli. Infect Immun. 2002; 70(5):2681-2689.
[Crossref] [Google scholar] [Pubmed]
- Marchès O, Ledger TN, Boury M, Ohara M, Tu X. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol Microbiol. 2003; 50(5):1553-67.
[Crossref] [Google scholar] [Pubmed]
- Reid SD, Herbelin CJ, Bumbaugh AC, Selander RK, Whittam TS .Parallel evolution of virulence in pathogenic. Nature. 2000; 406(6791):64-7.
[Crossref] [Gooogle scholar] [Pubmed]
- Dubreuil JD.Escherichia coli STb enterotoxin. Microbiology. 1997; 143(6):1783-95.
[Crossref] [Google scholar] [Pubmed]
- Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Bacteria–host communication: the language of hormones. Proc Natl Acad Sci. 2003; 100(15):8951-8956.
[Crossref] [Google scholar] [Pubmed]
- Microbiological assay of antibiotics. Indian pharmacopeia. 2022.
- Purohit CK K, Gokhale SB. A Text Book of Pharmacognosy. 2014.
- Bharskar G, Siddheshwar S. Formulation and in-vitro Evaluation of Topical Antimicrobial Preparation. Int J Pharm. 2022; 76:19-22.
- Hewitt W. Microbiological assay for pharmaceutical analysis: a rational approach. CRC press; 2003.
- Lorian V. Antibiotics in laboratory medicine. Lippincott Williams & Wilkins. 2005.
- Isenberg HD. Clinical microbiology procedures handbook. 2004.
- Cushnie TP, Hamilton VE, Chapman DG, Taylor PW, Lamb AJ. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J Appl Microbiol. 2007. 103(5):1562-7.
[Crossref] [Google scholar] [Pubmed]
- DeBlanc Jr HJ, Charache P, Wagner Jr HN. Automatic radiometric measurement of antibiotic effect on bacterial growth. Antimicrob Agents Chemother. 1972; 2(5):360-366.
[Crossref] [Google scholar] [Pubmed]
- Branson E (2001). Clinical relevance of minimum inhibitory concentrations (MICs). Aquaculture.196(3-4):289-296.
- Pinto TD, Lourenço FR, Kaneko TM. Microbiological assay of Gentamycin employing an alternative experimental design. Final Program. 2007.
[Crossref] [Google scholar] [Pubmed]
- Oden EM, Stander H, Weinstein MJ .Microbiological assay of gentamicin. Antimicrobial Agents and Chemotherapy.1963, 161:8-13.
[Crossref] [Google scholar] [Pubmed]
- Washington 2nd JA, Ritts Jr RE, Martin WJ. In vitro susceptibility of gram-negative bacilli to gentamicin. Mayo Clin Proc. 1970;45(2): 146-149.
[Crossref] [Google scholoar] [Pubmed]
- Wiegand I, Hilpert K, Hancock RE .Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008; 3(2):163-175.
[Crossref] [Google scholar] [Pubmed]
Citation: Patel P, Patel I, Kachare S, Maurya P (2024). Comparative Investigation of Commercially Available Anti-Bacterial Creams for their Efficacy Against Different Bacterial Strains. J Bacteriol Parasitol. S27:100.
Copyright: © 2024 Patel P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.