Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 16, Issue 6
Comparative Bioavailability Study of Rybelsus (Semaglutide) 3 mg Tablets of Novo Nordisk Versus Ozempic (Semaglutide) 2 mg/1.5 ml (1.34 mg/ml) Solution for Injection of Novo Nordisk under Fasting Condition in Healthy Subjects
Arjun Arumugam*, Geetha Lakshmi, Srinivas Gopineedu and Nageswara RaoReceived: 09-Nov-2024, Manuscript No. JBB-24-27495; Editor assigned: 11-Nov-2024, Pre QC No. JBB-24-27495 (PQ); Reviewed: 25-Nov-2024, QC No. JBB-24-27495; Revised: 02-Dec-2024, Manuscript No. JBB-24-27495 (R); Published: 09-Dec-2024, DOI: 10.35248/0975-0851.24.16.606
Abstract
Background: The human GLP-1 receptor agonist, semaglutide, functions by binding to albumin, which is facilitated by modifying position 26 lysine with a hydrophilic spacer and a C18 fatty di-acid. In this study, Novo Nordisk's Ozempic (Semaglutide/1.5 mL (1.34 mg/mL) solution for injection and Rybelsus (Semaglutide) 14 mg tablets) were compared to determine the pharmacokinetic parameters of Semaglutide after oral and subcutaneous administration in healthy subjects. Through the Flash Glucose Monitoring System with a sensor, we evaluated the pharmacodynamic response of semaglutide the absorption of glucose into the blood when the drug was administered in two different routes, using Novo Nordisk's Semaglutide 3 mg tablets in healthy subjects. To evaluate pharmacokinetics of Semaglutide injection and tablet and to assess the applicability of an LCMS/MS quantification method.
Materials and methods: Dose and mode of administration, batch, expiry date, Ozempic (Semaglutide) 2 mg/1.5 mL (1.34 mg/mL) solution for injection, 0.37 mL × 1.34 mg (0.5 mg), subcutaneous injection in sitting posture under fasting condition, MP5E309, July, 2025. Dose and mode of administration, lot, expiry date, Rybelsus (Semaglutide) 3 mg tablets, 01 × 3 mg, oral with 240 ± 02 mL of water in sitting posture under fasting condition, MS6GG22, Feb, 2024. The 90% confidence intervals were evaluated for comparison and to evaluate the absolute bioavailability of Cmax and AUC0-t. The 90% confidence intervals for Cmax and AUC0-t were 14.78%-58.01% and 4.51%-17.65% respectively.
Conclusion: The study was conducted as a proof-of-concept study to evaluate the pharmacokinetics of semaglutide when administered-subcutaneously and when administered as a tablet through the oral route. The pharmacokinetics obtained are similar to the reported values and when compared, the absolute bioavailability is about 1%-2% between these two products.
Keywords
Semaglutide; Bioavailability; Pharmacokinetic; Healthy subjects
Introduction
Semaglutide is a human GLP-1 receptor agonist (or GLP-1 analog). The main protraction mechanism of semaglutide is albumin binding, facilitated by modification of position 26 lysine with a hydrophilic spacer and a C18 fatty di-acid [1-3].
Furthermore, semaglutide is modified in position 8 to provide stabilization against degradation by the enzyme dipeptidylpeptidase 4 (DPP-4). A minor modification was made in position 34 to ensure the attachment of only one fatty di-acid [4,5]. Each Ozempic solution for injection contains 2 mg/1.5 mL (1.34 mg/ mL) of Semaglutide, and each Rybelsus tablet contain 14 mg of Semaglutide. Its molecular formula is C187H291N45O59, and it has a molecular weight of 4113.58 g/mol.
Materials and Methods
The study design was an open-label, balanced, randomized, single dose, two treatments, single period, parallel, comparative bioequivalence study under fasting condition. The study was conducted on six male subjects in the age group of 22 to 42 years who met the study eligibility criteria. The study was conducted with subcutaneous injection and orally administered tablets [6]. From the six subjects, three subjects received subcutaneous injection and the other three received oral tablets. Blood samples were collected up to 96 hours and 144 hours post-dose, respectively, for subjects dosed with tablet and injection. These samples were used for measurement of pharmacokinetic parameters of both the products. The pharmacodynamic response of the drug when administered in two different routes was also evaluated. Safety evaluation was done by assessing clinical examinations, vital signs assessments, clinical laboratory parameters, and monitoring the subject’s wellbeing, symptoms and signs for adverse events. A validated LC-MS/ MS method was used to determine the plasma concentrations of semaglutide [7].
Volunteers
A total of six subjects were selected and allowed to participate in the study.
Inclusion criteria for this study were:
a) Healthy male literate volunteers of 18 to 45 years (both years inclusive) and weight >50 kg.
b) Healthy volunteers within acceptable body mass index as deemed by the investigator.
c) Healthy volunteers as evaluated by medical history, vitals and general clinical examination.
d) Normal or clinically insignificant biochemical, hematological, urine and serology parameters.
e) Normal or clinically insignificant ECG.
f) Negative urine test for drugs of abuse, alcohol breath analysis.
g) Volunteers who are willing to use acceptable methods of contraception.
h) Volunteers who can give written informed consent and communicate effectively [8,9].
Exclusion criteria for this study were:
a) History of any major surgical procedure in the past 3 months.
b) History of any clinically significant cardiac, gastrointestinal, respiratory, hepatic, renal, endocrine, neurological, metabolic, psychiatric and hematological disorders.
c) History of chronic alcoholism/chronic smoking or drug abuse.
d) Volunteers with known hypersensitivity to semaglutide or any of the excipients.
e) History of consumption of tobacco-containing products within 48 hours prior to the proposed time of dosing.
f) Volunteers who are positive for hepatitis B surface antigen, anti-hepatitis C antibody, treponemal antibodies and Human Immunodeficiency Virus (HIV 1 and 2) antibodies.
g) Volunteers with a history of intake of drugs or any prescription drug or Over-the-Counter (OTC) drugs/vaccines (including COVID-19 vaccines) within 14 days or a significant duration as deemed by the investigator that potentially modify the kinetics/ dynamics of semaglutide or any other medication judged to be clinically significant by the investigator.
h) History of consumption of grapefruit and/or its products within 10 days prior to the start of study.
i) Volunteer who had participated in any other clinical study or who had bled during the last 3 months before check-in.
j) History of consumption of one or more of the below, 48 hours prior to dosing: Xanthine containing food or drinks such as cola, chocolate, coffee or tea, citrus fruits or items (lime, lemon, and orange), alcohol and any other food or beverage known to have interactions as deemed by the investigator [10,11].
Study design
An open-label, balanced, randomized, single dose, two treatments, single period, parallel, comparative bioequivalence study of Rybelsus (Semaglutide) 3 mg tablets of Novo Nordisk and Ozempic (Semaglutide) 2 mg/1.5 mL (1.34 mg/mL) solution for injection of Novo Nordisk in healthy, adult, human male subjects under fasting conditions.
Study subjects received either an injection or a tablet per the randomization schedule (Figure 1).
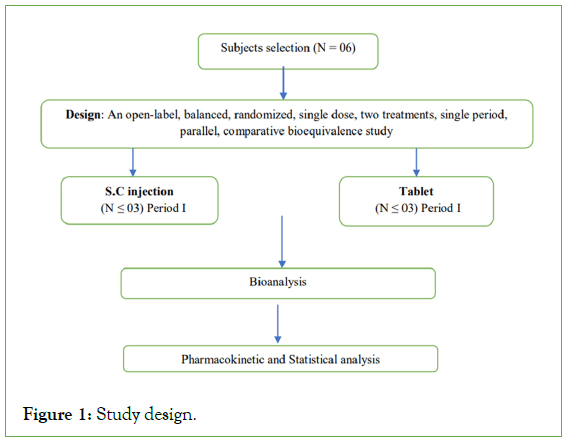
Figure 1: Study design.
Drug administration
After an overnight fasting of 8 hours, the subjects were administered a single oral dose of either of the products. The injection was administered to three subjects as directed in the product information. The tablet was administered with 240 mL of water. Compliance with dosing following drug administration was assessed by examination of the oral cavity. Drug dosing details were recorded in the individual case report forms. The principal investigator and/ or sub-investigator/physician were present throughout the conduct of the study. All the subjects remained in sitting posture for 2 hours after dosing and were permitted to walk for short intervals of time for reasons such as but not limited to the following: Natural exigencies, blood sampling and vitals measurement activity. The subjects received standard food approximately at 04.00, 08.00 and 12.00 hours post-dose with time flexibility of more than 15 minutes.
Blood sampling
Tablet: A total of 22 blood samples of 5 ml each at 00.00 (predose), 00.17, 00.33, 00.50, 00.75, 01.00, 01.50, 02.00, 02.50, 03.00, 04.00, 05.00, 06.00, 07.00, 08.00, 10.00, 12.00, 24.00, 34.00, 48.00, 72.00, and 96.00-hours post-dose, were collected for measurement of pharmacokinetic parameters in each period.
Injection: A total of 21 blood samples of 5 ml each at 00.00 (predose), 02.00, 04.00, 08.00, 12.00, 24.00, 28.00, 32.00, 36.00, 40.00, 44.00, 48.00, 52.00, 56.00, 60.00, 64.00, 72.00, 84.00, 96.00, 120.00 and 144.00-hours post-dose were collected for measurement of pharmacokinetic parameters in each period. The pre-dose samples were collected two hours before the drug administration.
The post-dosage in-house samples were collected within 2 min of the scheduled time, and the ambulatory samples were collected within ± 1 hours from the scheduled time. Any delay from these was recorded as protocol deviation. The samples were collected from intravenous cannulas or by direct vein puncture into pre-labeled K3EDTA vacutainers. The samples were then centrifuged at 3500 rpm for 10 min at 4°C. The plasma was separated into two aliquots: 1 ml in the first aliquot and remaining in the second aliquot. The K3EDTA vacutainers and polypropylene tubes were pre-labeled with the subject number, study number, study period, time point and aliquot number. The samples were stored at -70°C in an ultralow temperature freezer. The plasma samples were quantified using validated bioanalytical methods.
Glucose monitoring
A flash glucose monitoring system was used to monitor the glucose levels through a sensor. A sensor was fixed to the back of the arm and the glucose levels were monitored for 3 days.
Analytical method
Liquid-liquid extraction was chosen for sample preparation to extract the analyte of semaglutide. A validated LC-MS/MS bioanalytical method was used for the estimation of semaglutide in plasma. Bioanalytical method validation was done as per the guidance. All samples from one subject were analyzed with the same single standard curve. Sample concentration above the upper limit of the standard curve from the validated range was analyzed by diluting the sample with a drug-free biological matrix and assaying.
Pharmacokinetic parameters and statistical analysis
The analysis of variance (ANOVA) was performed on the Lntransformed data of Cmax and AUC0-t using PROC GLM of SAS® (version 9.4) software.
The ANOVA was performed on the Ln-transformed Cmax and AUC0-t parameters. The least-squares mean ratios, 90% CI for Cmax and AUC0-t of Semaglutide for both the products.
Safety analysis
Safety was assessed via continuous health monitoring and scheduled recording of safety measurements throughout the study. Staff monitored and recorded the pulse rate, oral temperature, blood pressure and subjects’ well-being during check-in at 00.00 (pre-dose), 03.00, 09.00, 32.00, 54.00, 74.00 and 95.00-hours post-dose, poststudy and at the discretion of the clinical staff. Temperature will be recorded during check-in, before check-out (95.00 hours post-dose), post-study, along with other vital parameters, and at the discretion of clinical staff. The pre-dose (00.00 hours) vital parameters were recorded within two hours before drug administration and the post-dose vitals with a flexibility of ± 30 min of each time point.
General examination and systemic examination of subjects were performed during check-in of each period by the physician/ investigator/sub-investigator to ensure safety. Subjects were instructed to inform clinic personnel of any untoward medical symptoms and/or events that arose during the course of the study.
The principal investigator/sub-investigator/study physician evaluated the relationship of all adverse events to the study drugs. The adverse events were graded as mild, moderate, or severe as per standard operating procedure. The principal investigator/ sub-investigator also evaluated the subjects for subsequent dosing. Post-clinical laboratory tests (hematology and serum chemistry) and post-study general and systemic examination, including vital sign measurements (blood pressure, pulse rate and temperature), were performed.
Results
Pharmacokinetics and statistics
In the present study, six subjects participated, all subjects completed the study. The concentrations of all the subjects were subjected to pharmacokinetic and statistical analysis. The plasma concentration vs. time curve of both the products is presented in (Figures 2 and 3). The geometric mean ratios, 90% CI, and power of variation of both the products for Ln transformed pharmacokinetic parameters Cmax and AUC0-t for semaglutide are presented in Tables 1 and 2 includes the pharmacokinetics of Semaglutide after oral and subcutaneous administration. The glucose levels of all subjects at various times of a day were plotted and presented in (Figure 4).
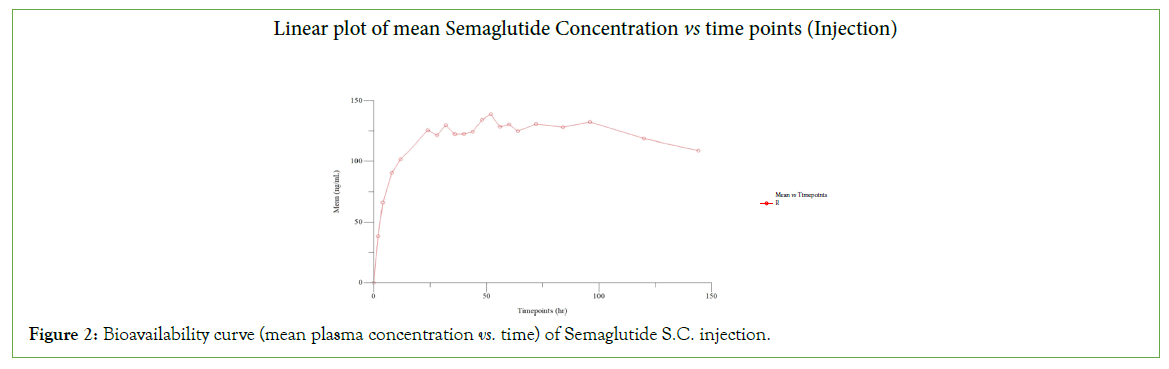
Figure 2: Bioavailability curve (mean plasma concentration vs. time) of Semaglutide S.C. injection.
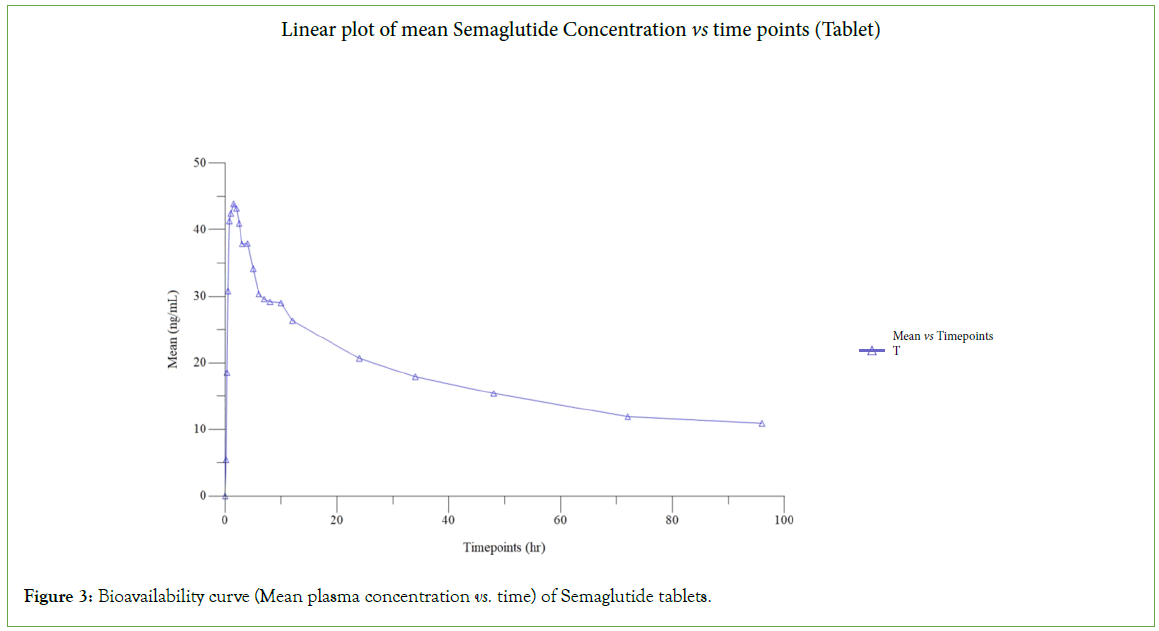
Figure 3: Bioavailability curve (Mean plasma concentration vs. time) of Semaglutide tablets.
| Pharmacokinetic parameters | Test geometric mean | Reference geometric mean | Test/Reference ratio | 90% Confidence interval for test vs. reference | Power of ANOVA | Inter subject cv |
|---|---|---|---|---|---|---|
| LN_Cmax | 41.5014 | 141.7167 | 29.28 | 14.78%-58.01% | 0.1122 | 40.83 |
| LN_AUCT | 1531.6591 | 17174.648 | 8.92 | 4.51%-17.65% | 0.1123 | 40.77 |
Table 1: The geometric mean ratios, 90% CIs, power and inter-subject coefficient of variation of tablet and injection for Ln transformed pharmacokinetic parameters Cmax and AUC0-t for semaglutide are presented below.
| Parameter | Ozempic (Semaglutide) (Mean ± SD) | Rybelsus (Semaglutide) (Mean ± SD) |
|---|---|---|
| Cmax (ng/mL) | 141.942 ± 9.835 | 46.236 ± 27.619 |
| AUC0-t (ng.hr/mL) | 17208.388 ± 1306.624 | 1698.962 ± 969.003 |
| AUC0-∞ (ng.hr/mL) | 63591.111 ± 27007.229 | 2593.373 ± 1421.879 |
| tmax (hr) | 60.00 (48.00-96.00) | 0.75 (0.50-2.00) |
| Kel (hr-1) | 0.002 ± 0.001 | 0.012 ± 0.001 |
| T1/2 (hr) | 298.577± 110.130 | 57.588 ± 4.510 |
| AUC_%Extrap (%) | 71.673 ± 10.053 | 34.733 ± 3.031 |
Table 2: Summary of pharmacokinetic parameters for semaglutide.
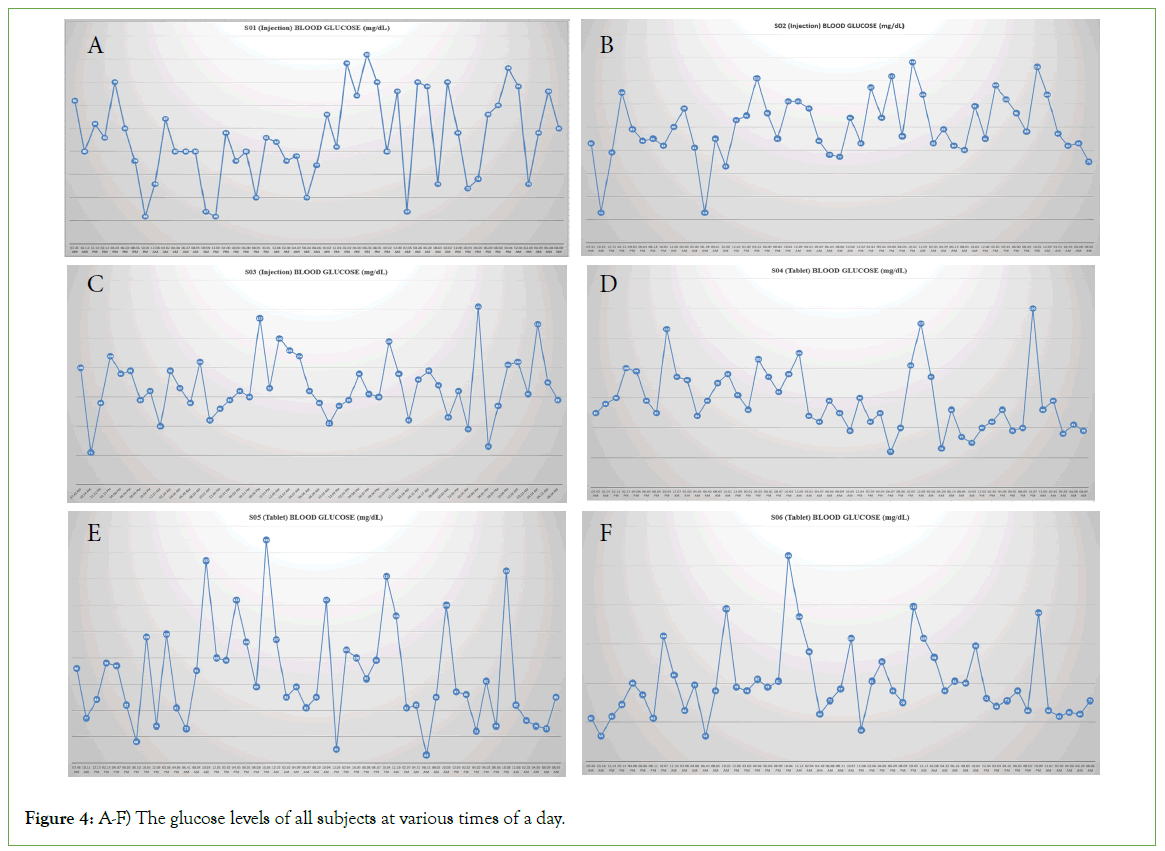
Figure 4: A-F) The glucose levels of all subjects at various times of a day.
Brief summary of adverse events
Safety was evaluated throughout the study and there were no adverse events. No serious adverse events were reported in this study. Thus, both the test and reference products were well tolerated.
Discussion
This study was conducted as a proof-of-concept study to understand the pharmacokinetics of the subcutaneous injection and the orally administered tablet as a single dose to healthy subjects. Considering the long half-life of the drug, and to assess the suitability of an LCMS/MS application to quantify the drug levels parallel design was adopted.
Blood samples were collected at pre-specified points after dosing to obtain a pharmacokinetic profile of both the products. Continuous glucose monitoring was also performed for all the subjects to observe the effect of the drug on glucose absorption and metabolism. This continuous glucose monitoring was performed using a flash glucose monitoring system through a sensor attached at the back of the upper arm. This enabled monitoring of glucose continuously for 4 days.
The study results obtained were satisfactory, and the data obtained provides an overview of the pharmacokinetics and pharmacodynamic properties of both drugs after a single dose administration. Though the study was not intended to evaluate the bioequivalence of both the products, an Analysis of Variance (ANOVA) of Cmax and AUC parameters were evaluated. As reported [4], the bioavailability of the orally administered tablet is about 1% of the injection administered subcutaneously, despite the dose being only about 16% of the tablet’s dose. The effect on the glucose levels is evidently seen for both products. No appreciable spikes of glucose concentration were observed for 3 days after administration for both products.
Conclusion
The pharmacokinetics of semaglutide administered subcutaneously and orally were measured with the application of a suitable LCMS/ MS method. Though a relatively low dose was administered orally, semaglutide could be quantified in all the samples. The interchangeability between two dosage forms could not be studied considering limited sample size. This study will be helpful in designing further studies in a large sample size with different objectives.
Informed Consent
The protocol and Informed Consent Forms (ICFs) were reviewed and approved by an independent ethics committee. Independent Ethics Committee, Chennai (Ethics Committee re-registration No. ECR/111/Indt/TN/2013/RR-19 issued under Rule 122D of the Drugs and Cosmetics Rules, Ministry of Health and Family Welfare, Directorate General of Health Services, Office of the Drugs Controller General (India), Central Drugs Standard Control Organization), before study initiation. The study-informed consent documents were distributed to all the eligible subjects. The consent documents were read and explained by the staff. Subjects were given adequate time to read and understand the consent documents. Illiterate volunteers were explained vocally by the investigator in their vernacular language. Individual counseling was then given to the willing volunteers by the investigator in private, and any questions and concerns were addressed before obtaining consent. Before the initiation of the study, written informed consent was acquired from each of them, and the study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and national regulatory requirements.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement
All technical, clinical, and analytical execution of the bioequivalence studies was performed independently by Azidus Laboratories LTD.
Reference
- Chao AM, Tronieri JS, Amaro A, Wadden TA. Clinical insight on semaglutide for chronic weight management in adults: Patient selection and special considerations. Drug Des Devel Ther. 2022:4449-4461.
[Crossref] [Google Scholar] [PubMed]
- Snitker S, Andersen A, Berg B, van Marle S, Sparre T. Comparison of the injection-site experience of the starting doses with semaglutide and dulaglutide: A randomized, double‐blind trial in healthy subjects. Diabetes Obes Metab. 2021;23(6):1415-1419.
[Crossref] [Google Scholar] [PubMed]
- Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
[Crossref] [Google Scholar] [PubMed]
- Jensen L, Kupcova V, Arold G, Pettersson J, Hjerpsted JB. Pharmacokinetics and tolerability of semaglutide in people with hepatic impairment. Diabetes Obes Metab. 2018;20(4):998-1005.
[Crossref] [Google Scholar] [PubMed]
- Perry R. Perspectives on the bioequivalence and therapeutic equivalence of generic formulations: An overview of the landscape. Clin Ther. 2010;32(10):1796-1797.
[Crossref] [Google Scholar] [PubMed]
- Chen ML, Shah V, Patnaik R, Adams W, Hussain A, Conner D, et al. Bioavailability and bioequivalence: An FDA regulatory overview. Pharm Res. 2001;18:1645-1650.
[Crossref] [Google Scholar] [PubMed]
- World Medical Association. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191-2194.
[Crossref] [Google Scholar] [PubMed]
- Singh J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J Pharmacol Pharmacother. 2015;6(3):185-187.
[Crossref] [Google Scholar] [PubMed]
- statistical Approaches to Establishing Bioequivalence. 2001.
- Product-Specific Guidances for Generic Drug Development. 2022.
- Clinical Pharmacology and Biopharmaceutics Review(s). 2012.
Citation: Arumugam A, Lakshmi G, Gopineedu S, Rao N (2024). Comparative Bioavailability Study of Rybelsus (Semaglutide) 3 mg Tablets of Novo Nordisk Versus Ozempic (Semaglutide) 2 mg/1.5 ml (1.34 mg/ml) Solution for Injection of Novo Nordisk Under Fasting Condition in Healthy Subject. J Bioequiv Availab. 16:606.
Copyright: © 2024 Arumugam A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

