Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 16, Issue 2
Comparative Bioavailability of Two Tablet Formulations of Amifampridine with and without Food, and the Impact of Acetylator Status on the Pharmacokinetic Food Effect
Keith D. Gallicano1*, Gary Ingenito2, Ardeshir Khadang3 and Peter Boldingh32Catalyst Pharmaceuticals, Inc., Coral Gables, Florida, USA
3AXIS Clinicals, 1711 Center Avenue West, Dilworth, Minnesota, 56529-1342, USA
Received: 15-Apr-2024, Manuscript No. JBB-24-25460; Editor assigned: 17-Apr-2024, Pre QC No. JBB-24-25460 (PQ); Reviewed: 01-May-2024, QC No. JBB-24-25460; Revised: 01-May-2024, Manuscript No. JBB-24-25460 (R); Published: 15-May-2024, DOI: 10.35248/0975-0851.24.16.566
Abstract
Background: Previous research has not evaluated whether the two pharmaceutically non-equivalent tablet products containing amifampridine, Ruzurgi® (amifampridine) and Firdapse® (amifampridine phosphate), are bioequivalent under fasted and fed conditions, and if acetylator status influences the magnitude of the food effect. Therefore, we compared the bioavailability of amifampridine tablet 10 mg relative to that of amifampridine phosphate tablet 10 mg (base equivalent) in the fed state following consumption of a high-fat meal and in the fasted state, and investigated the effect of food intake on the pharmacokinetics of amifampridine and its inactive 3-N-acetyl metabolite in subjects evaluated for slow or rapid/intermediate N-acetyltransferase 2 (NAT2) metabolizer status.
Methods: Twenty (20) healthy, adult male and female volunteers were enrolled in this open-label, randomized, four-treatment, two-sequence, four-period, crossover, single-dose, oral comparative bioavailability and food-effect study. Eighteen (18) individuals (Male: 10; Female: 8; Slow NAT2 metabolizer: 9; Rapid/intermediate NAT2 metabolizer: 9), completed all four periods. Plasma concentrations and pharmacokinetic characteristics of amifampridine and 3-N-acetyl amifampridine were determined by LC-MS/MS. Safety profiles of the two products were assessed from adverse events monitoring, medical examinations, and clinical laboratory tests.
Results: Compared to rapid/intermediate acetylators, slow acetylators had statistically significant 5.5- to 8.9-fold higher amifampridine Cmax and AUC and a 1.8-fold longer t½z (1.48 to 2.62 hours), and 22%-31% lower 3-N-acetyl amifampridine AUC and Cmax. Metabolite t½z values were similar between the two phenotypes (Rapid/intermediate: 3.50 hours; Slow: 3.66 hours). Under fasted and fed conditions, the 90% confidence intervals for the least-squares geometric mean ratios of the Test (Ruzurgi®) to Reference (Firdapse®) treatments were within the standard equivalence range (80%, 125%) for Cmax, AUC0-t and AUC0-∞ parameters for amifampridine and metabolite. For rapid/intermediate acetylators, the high-fat meal significantly decreased amifampridine AUC by 34%-40%, and Cmax by 69%. For slow acetylators, AUC was unaffected by food but Cmax decreased by 39%. The single oral doses were well tolerated under fasted and fed conditions.
Conclusion and implications: Peak and total plasma exposures of amifampridine and its metabolite were equivalent between the two products following a single 10-mg dose in either the fasted or fed state. Therefore, dosing regimens of Ruzurgi® and Firdapse® can be considered interchangeable in the fasted and fed states.
A high-fat meal decreased peak and total plasma exposures of amifampridine and 3-N-acetyl amifampridine, but the effect was more pronounced on amifampridine for rapid/intermediate acetylators, indicating the importance of knowing an individual’s acetylator status to avoid potential underdosing either product with a high-fat meal.
Keywords
Amifampridine; Arylamine N-acetyl transferase; Food effects; Lambert-Eaton myasthenic syndrome; Pharmacokinetics; Bioequivalence
Introduction
Oral drug products containing the potassium channel blocker amifampridine (3,4-diaminopyridine), either as the phosphate salt (Firdapse®) or free base (Ruzurgi®), have been prescribed for the symptomatic treatment of patients with Lambert-Eaton Myasthenic Syndrome (LEMS), which is a very rare and severely debilitating neuromuscular disorder. Although bioequivalence was evaluated between different galenical forms of the phosphate salt (tablet) and free base (capsule) of amifampridine in the fasted state [1], the bioequivalence between Firdapse® and Ruzurgi®, which are two pharmaceutical alternative tablet products that contain different forms of active pharmaceutical ingredient, has not been established under fasted and fed conditions.
The human drug-metabolizing enzyme N-acetyltransferase (NAT) consists of two different isoenzymes (NAT1 and NAT2), both of which are encoded by two highly polymorphic genes located on chromosome 8, and are responsible for N-acetylation of arylamines and arylhydrazines and O-acetylation of arylhydroxylamines [2]. Amifampridine is metabolized by both NAT1 and NAT2 but NAT2 has an eightfold higher metabolic rate over NAT1 [3].
Point mutations in the NAT2 gene lead to decreased acetylation activity [2]. The four main haplotype series of NAT2 known to be associated with an altered enzyme function are NAT2*5, NAT2*6, NAT2*7 and NAT2*14, all of which result in reduced function compared to the wild-type allele NAT2*4 [4]. The NAT2 acetylator phenotype is classified as either ultra-slow, slow, intermediate or rapid [4]. Individuals homozygous for NAT2*4 are considered rapid acetylators, those with one rapid and one slow allele are considered intermediate acetylators, and those with no rapid alleles are considered slow acetylators. Additionally, individuals genotyped as NAT2*6/*6, *6/*7 or *7/*7 can be categorized into the ultra-slow acetylator phenotype in N-acetylation of hydralazine, phenelzine and sulfamethazine [4]. NAT2 rapid acetylator phenotypes are more prevalent among Japanese, Chinese, Korean and Thai whereas NAT2 ultra-slow and slow acetylator phenotypes are more common in North American and European populations [4-6].
Amifampridine pharmacokinetics is characterized by a large difference in plasma exposures of amifampridine between slow and rapid NAT2 phenotypes with normal renal and hepatic function, for which AUC and Cmax values were 5.5-fold and 5.0-fold higher, respectively, in slow NAT2 acetylators relative to rapid NAT2 acetylators [7]. Plasma concentrations of the inactive 3-N-acetyl metabolite are higher than those for amifampridine in both acetylator groups [3,7].
The study by Haroldsen, et al., demonstrated that a high-fat, high-calorie meal reduced peak and total plasma exposures of amifampridine by 44% (Cmax) and 20% (AUC), respectively, and prolonged tmax by 0.7 hr relative to the fasted state following oral administration of amifampridine phosphate salt to healthy volunteers of unknown NAT2 phenotype [8]. Although the administration instructions in the 2023 U.S. FDA Prescribing Information for Firdapse® state that the drug product can be taken without regard to food, previous research has not evaluated if acetylator status influences the magnitude of the food effect despite a proposal that rapid acetylators be informed to take 3,4-diaminopyridine without food [1].
Therefore, the two objectives of the study were to compare the bioavailability of amifampridine tablet 10 mg relative to that of amifampridine phosphate tablet 10 mg (base equivalent) in the fed state following consumption of a high-fat meal and in the fasted state, and investigate the effect of food intake on the pharmacokinetics of amifampridine and its inactive 3-N-acetyl metabolite in subjects evaluated for slow or rapid/intermediate NAT2 metabolizer status to determine if there is a differential effect of food by acetylator status.
Methodology
Ethical conduct of the study
The study protocol, Informed Consent Form, and other supporting documents for the study drug were reviewed and approved by an independent ethics committee (Advarra Institutional Review Board, Columbia, Maryland USA). The study was conducted at AXIS Clinicals, Dilworth, Minnesota USA in compliance with the protocol and in accordance with good clinical practice as per the principles of the Declaration of Helsinki and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E6 Guideline for Good Clinical Practice in effect at the time of study conduct.
Volunteer population
Twenty (20) healthy adults (11 males and 9 non-pregnant, non- lactating females) aged 18 years and older with a Body Mass Index (BMI) ranging between 18.00 to 32.49 kg/m2, inclusive, were selected according to inclusion and exclusion criteria. Participants were assessed to be in healthy condition based on a pre-study medical examination including medical history, medication history, complete physical examination, vital signs (blood pressure, pulse rate, respiration rate, and temperature), 12-lead electrocardiogram, and clinical laboratory tests (including hematology, chemistry, urinalysis, test for drugs of abuse, alcohol breath analysis, serology, serum pregnancy test (for all females), and N-acetyltransferase gene 2 (NAT2) genetic test for the assessment of acetylator genotype and assignment of acetylator phenotype) that were done during screening.
Study design
The study design was an open-label, randomized, four-treatment, two-sequence, four-period, crossover, single-dose, oral comparative bioavailability and food-effect study in which each participant received the two formulations in the fasted and fed states with at least three days’ washout between treatment administrations. Participants were stratified based on slow or rapid/intermediate NAT2 metabolizer status. The Test product was Ruzurgi® (amifampridine) tablet 10 mg (Jacobus Pharmaceutical Company, Inc., Plainsboro, NJ USA), and the Reference product was Firdapse® (amifampridine phosphate) tablet 10 mg base equivalent (Catalyst Pharmaceuticals, Inc., Coral Gables, FL USA).
The four treatments were Test (A) and Reference (B) administered as a single 10-mg tablet with approximately 240 mL of ambient drinking water at 30 minutes after start of consumption of a high-fat (~50% of total caloric content), high-calorie (~1000 kilocalories) breakfast and Test (C) and Reference (D) administered as a single 10-mg tablet with approximately 240 mL of ambient drinking water following at least a 10-hr fast before dosing. The breakfast consisted of two eggs fried in butter, two strips of bacon, two slices of toast with butter, four ounces of hash brown potatoes, and eight ounces of whole milk. The two-sequence randomization scheme was ABCD and BADC. The randomization was balanced for NAT2 acetylator status across volunteers and within each sequence. That is, 10 NAT2 slow and 10 NAT2 rapid/intermediate acetylators, 5 each per sequence.
NAT2 genotyping
NAT2 genotyping was conducted by Invitae Corporation (San Francisco, CA USA). Genomic DNA was isolated from blood containing dipotassium ethylenediaminetetraacetic acid (K2EDTA anticoagulant) using the Promega Maxwell HT 96 gDNA isolation system (Promega, Madison, WI) on the KingFisher Flex automated extraction instrument (Thermo Scientific, Waltham, MA). NAT2 genotyping was then performed for the following alleles *4 (wild type), *5A-E, *5G, *5J, *6A-C, *6E, *7A, *7B, *11, *12A-D, *13, *14A-G and *19 using the MassARRAY Analyzer 4 Instrument (MAA4, Agena, San Diego CA). The method is a laboratory developed test that consisted of an initial locus-specific polymerase chain reaction, followed by single base extension using mass-modified dideoxynucleotide terminators of an oligonucleotide primer that anneals immediately upstream of the polymorphic site of interest. Using MALDI-TOF mass spectrometry, the distinct mass of the extended primer was then used for identification of the wild-type and variant sequences at each genomic position that was interrogated.
NAT2 genotyping was performed to identify 10 individuals who are slow acetylators and 10 individuals who are rapid/intermediate acetylators of amifampridine. Intermediate acetylators were included in the class of rapid acetylators because rapid acetylators generally contain two wild-type (high-activity) alleles (*4/*4), or one wild-type and one variant allele (also termed intermediate) with a nucleotide substitution of NAT2, whereas a slow acetylator phenotype is observed for individuals with two germ-line copies of low-activity alleles.
Blood sampling
Sixteen (16) blood samples of 1 x 6 mL were collected in pre-labelled vacutainer tubes containing K2EDTA during each study period. A pre- dose (0.00 hour) blood sample was collected within 90 minutes before dosing in each period. Post-dose blood samples were collected at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10 and 12 hours in each period.
Bioanalytical analysis
Plasma sample (0.1 mL) was mixed with solutions of deuterium- labeled internal standards of amifampridine-d3 and 3-N-acetyl-d3 amifampridine in 50:50 water:acetonitrile (0.025 mL) and 30% ammonium hydroxide in water (0.025 mL). Amifampridine, 3-N-acetyl amifampridine, and internal standards were isolated from plasma by a liquid-liquid extraction with ethyl acetate (0.6 mL). Dried extracted samples were reconstituted in 10:90 water:acetonitrile (0.2 mL), chromatographically separated on a Waters Xbridge HILIC analytical column (50 x4.6 mm, 5 μm, 25°C) by a gradient mobile phase of 10 mM ammonium formate and 0.1% formic acid in water as mobile phase A and 100% acetonitrile as mobile phase B, and injected. The retention times for a 10 μL injection were 2.60 ± 0.50 min for amifampridine and amifampridine-d3 and 2.80±0.50 min for 3-N-acetyl amifampridine and 3-N-acetyl-d3 amifampridine.
Analyte detection was by mass spectrometry operated in the positive ion mode with tandem quadrupole mass filtering using an API 4500 LC-MS/MS equipped with turbo ion-spray. The setting for Multiple Reaction Monitoring (MRM) detection of each analyte consisted of a characteristic protonated precursor ion [M+H+] to product ion mass transition, as follows: Amifampridine, 110.0→66.0 Da; amifampridine-d3, 113.0→68.0 Da; 3-N-acetyl amifampridine, 152.0→83.0 Da; and 3-N-acetyl-d3 amifampridine, 155.0→84.0 Da. The linear dynamic range of the standard curve was 0.5 to 250 ng/mL for amifampridine and 1.5 to 750 ng/mL for metabolite. The Lower Limit of Quantitation (LLOQ) for the assay was 0.5 ng/mL for amifampridine and 1.5 ng/mL for metabolite.
The between-run (inter-run) precision (% CV) and accuracy (% Bias) of standards was assessed from the back-calculated concentrations from the standard curves of all validation runs. Each run had a standard curve at the front and end of the sample queue. Values ranged from 2.1% to 3.9% (% CV) and -3.2% to 3.6% (% Bias) for amifampridine standards and 1.7% to 9.1% (% CV) and -3.0% to 3.1% (% Bias) for 3-N-acetyl amifampridine standards.
The within-run (intra-run) and between-run precision and accuracy of Quality-Control (QC) samples was evaluated during validation by comparing measured concentrations of QC samples to their nominal values at four different concentration levels (LLOQ, low, medium and high) over the analytical runs. For amifampridine QC samples, values ranged from 0.7% to 8.9% and 1.5% to 9.6% for within-run and between-run precision % CV, respectively, and from -7.4 to 15.2% and -2.0% to 5.0% for within-run and between-run accuracy % Bias, respectively. For 3-N-acetyl amifampridine QC samples, values ranged from 0.5% to 15.9% and 1.8% to 11.9% for within-run and between- run precision % CV, respectively, and from -6.5% to 12.3% and 0.1% to 5.5% for within-run and between-run accuracy % Bias, respectively.
Pharmacokinetic methods
Plasma pharmacokinetic parameters were determined by noncompartmental analysis using Phoenix WinNonlin® version 8.3.4 (Certara USA Inc, Princeton, NJ, USA) for amifampridine and 3-N-acetyl amifampridine. The primary pharmacokinetic parameters for assessment of bioequivalence and food effect were Cmax, AUC0-t and AUC0-∞. Secondary pharmacokinetic parameters included tmax, first- order terminal disposition half-life (t½z), and molar Metabolic Ratio (MR) of metabolite AUC0-t to parent AUC0-t (MR AUC0-t, calculated as MR multiplied by 0.722, which is the ratio of molecular weight of parent to that of metabolite).
Statistical analysis
Comparative bioavailability of the Test and Reference products under fed and fasted conditions and the effect of food on the bioavailability of each respective formulation were evaluated by comparison of the ln- transformed pharmacokinetic parameters AUC0-t, AUC0-∞ and Cmax for amifampridine and 3-N-acetyl amifampridine. Treatment comparisons were based on Analysis of Variance (ANOVA) models using the Mixed Model procedure (PROC MIXED) of SAS® (Version 9.4) and an incomplete block design for the four pairwise comparisons (i.e., 2-at-a- time-principle with exclusion of the other two treatments).
For the relative bioavailability of the Test to Reference products under fed condition (Treatment A versus Treatment B) and fasted condition (Treatment C versus Treatment D) the ANOVA model contained the fixed effects of sequence, treatment, period, NAT2 status, and NAT2-status-by-treatment interaction, and random effect of subject- within-sequence-by-NAT2-status. For the effect of food on the Test product (Treatment A versus Treatment C) and the Reference product (Treatment B versus Treatment D) the statistical model contained the fixed effects of sequence, treatment, NAT2 status, and NAT2- status-by-treatment, and random effect of subject-within-sequence-by- NAT2-status. Period was excluded from the ANOVA model owing to confounding of period and treatment effects, considering the fed treatments always preceded the fasted treatments in this 4-period, 2-sequence design.
The food effect was also evaluated from the combined data of both products (i.e., Fed (A+B)/2 versus Fasted (C+D)/2) by first establishing there was no product-by-food-effect interaction (i.e., A-C = B-D). The product-by-food-effect interaction was evaluated by two methods: 1) from individual difference in ln-transformed data (individual (lnAlnC) versus individual (lnB-lnD)) and 2) from the Estimate statement in the ANOVA model for treatment effect using the treatment coefficients (1A+(-1) B+(-1) C+1D = 0). As the NAT2-status-by-treatment interaction was statistically significant at the 5% significance level (p<0.05) for the AUC and Cmax parameters for comparison of Fed (A+B)/2 versus Fasted (C+D)/2, the food effect was assessed for rapid/intermediate and slow acetylators by a subgroup analysis of the separate acetylator groups.
The NAT2-status-by-treatment interaction was excluded from the final model if it was not statistically significant at the 5% significance level (i.e., p≥0.05). Sequence was tested at the 10% significance level and NAT2 status was tested at the 5% significance level against the Type III mean square term for subject-within-sequence-by-NAT2- status as the error term. All other main effects were tested at the 5% level of significance against the residual error term. The sex effect was not included in the ANOVA model because the pharmacokinetic properties of amifampridine are not affected by sex [3,8].
Treatment effects were considered equivalent for each pharmacokinetic parameter if the 90% confidence interval for the ratio of least-squares geometric treatment means was within the standard equivalence range (80%, 125%).
Safety assessments
The safety and tolerability of the four treatments were assessed for the 20 enrolled volunteers, as they all received at least one treatment, based on medical review of adverse events and laboratory variables, vital signs, 12-lead ECGs, and physical examinations. All adverse events observed in the study were coded by using Medical Dictionary for Regulatory Activities version 25.1.
Results
Volunteer population
Of the 20 enrolled volunteers, 10 individuals with two low-activity NAT2 alleles (*5B/*5B (N=2), *6A/*6A (N=3), *5B/*6A (N=2), *5B/*5C (N=1), *5A/*5B (N=1) or *6A/*7B (N=1)) were classified as a slow acetylator phenotype, and 10 individuals with one or two high-activity *4 alleles (i.e., *4/*5B (N=2), *4/*5C (N=2), *4/*6A (N=1), *4/*7B (N=2) or *4/*4 (N=3)) were classified as intermediate and rapid acetylator phenotypes, respectively. Maximal balance was achieved between slow and rapid/intermediate acetylators within each of the 11 male (5 slow and 6 rapid/intermediate) and 9 female (5 slow and 4 rapid/intermediate) populations. The median age of the volunteers was 39 (Range: 23-71) years and median body mass index was 29.3 (Range: 23.2-31.9) kg/m2. The distribution of race was 80% White, 10% Black or African American, 5% Asian, and 5% American Indian/Alaska Native.
Eighteen (18) volunteers (Male: 10; Female: 8; Slow NAT2 metabolizer: 9; Rapid/intermediate NAT2 metabolizer: 9), completed all four periods of the study and were included in the comparative statistical analysis of pharmacokinetic parameters. Two (2) individuals, one slow acetylator (male) and one rapid/intermediate acetylator (female), were discontinued by the Investigator after the first period; their pharmacokinetic data were included in summary statistics in (Table 1), but were excluded from the statistical analysis comparing treatment effects (Tables 2-5) because they did not complete two periods for at least one of the four statistical comparisons (A versus B, C versus D, A versus C, or B versus D).
| Treatment | Test Fed | Reference Fed | Test Fasted | Reference Fasted | ||||
|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | |||||
| Acetylator Phenotype (N) | SA (9) |
RIA (10) |
SA (10) |
RIA (9) |
SA (9) |
RIA (9) |
SA (9) |
RIA (9) |
| Amifampridine mean (SD) pharmacokinetic parameters; tmax: median (min, max) | ||||||||
| AUC0-t, h·ng/mL | 69.5 (24.7) | 6.18 (3.39) | 63.1 (20.6) | 5.90 (2.86) | 70.4 (20.6) | 11.1 (5.89) | 69.3 (18.7) | 10.0 (4.49) |
| AUC0-∞, h·ng/mL | 73.0 (25.5) | 7.54 (3.81)a | 65.9 (21.4) | 7.40 (3.00)a | 73.1 (20.7) | 12.6 (6.17) | 72.3 (19.1) | 11.4 (4.72) |
| Cmax, ng/mL | 23.4 (11.0) | 3.62 (2.49) | 27.0 (10.5) | 2.90 (0.93) | 39.4 (12.8) | 10.6 (5.31) | 41.8 (16.9) | 10.4 (3.02) |
| tmax, h | 1.00 (0.50-1.50) | 0.75 (0.25-4.00) | 1.15 (0.75-2.50) | 1.53 (0.50-4.00) | 0.50 (0.25-0.57) | 0.50 (0.25-0.75) | 0.50 (0.50-0.75) | 0.50 (0.30-1.00) |
| t½z, h | 2.82 (1.01) | 1.60 (0.45) | 2.49 (0.52) | 1.33 (0.36) | 2.67 (0.43) | 1.54 (0.40) | 2.75 (0.55) | 1.63 (0.43) |
| 3-N-acetyl amifampridine mean (SD) pharmacokinetic parameters; tmax: median (min, max) | ||||||||
| AUC0-t, h·ng/mL | 475 (107) | 615 (72.8) | 462 (93.1) | 599 (105) | 562 (114) | 696 (99.8) | 540 (120) | 688 (107) |
| AUC0-∞, h·ng/mL | 545 (134) | 694 (105) | 529 (115) | 678 (130) | 636 (143) | 770 (116) | 616 (149) | 765 (126) |
| Cmax, ng/mL | 84.2 (15.2) | 124 (19.0) | 89.0 (18.3) | 118 (29.8) | 119 (25.1) | 179 (42.6) | 120 (28.4) | 179 (26.0) |
| tmax , h | 2.00 (0.75-3.00) | 1.50 (0.78-5.00) | 2.00 (1.00-3.00) | 2.50 (1.25-5.00) | 1.03 (0.75-1.50) | 0.75 (0.75-1.25) | 1.03 (0.75-1.50) | 0.75 (0.75-1.50) |
| t½z , h | 3.79 (0.64) | 3.58 (0.43) | 3.59 (0.47) | 3.40 (0.43) | 3.69 (0.61) | 3.51 (0.31) | 3.83 (0.27) | 3.60 (0.47) |
| MR AUC0-t | 5.47 (2.10) | 91.3 (45.7) | 5.75 (1.90) | 86.2 (36.6) | 6.20 (2.10) | 56.1 (25.8) | 5.96 (1.84) | 57.1 (20.8) |
Note: n: Sample size; SA: Slow Acetylator; RIA: Rapid/Intermediate Acetylator; SD: Standard Deviation; MR: (Molar) Metabolic Ratio. a N=9 for Treatment A and N=8 for Treatment B, as one RIA individual had no evaluable AUC0-∞ data for Treatments A and B.
Table 1: Summary of plasma pharmacokinetic parameters of amifampridine and 3-N-acetyl amifampridine for slow (SA) and Rapid/Intermediate (RIA) NAT2 metabolizers by treatment (A, B, C and D).
Amifampridine and 3-N-acetyl amifampridine pharmacokinetic results under fasted and fed conditions for the two products
Mean (SD) and median (minimum, maximum) plasma pharmacokinetic parameters of volunteers separated by acetylator phenotype are summarized for each treatment in (Table 1) for amifampridine and 3-N-acetyl amifampridine.
Comparative bioavailability under fasted and fed conditions
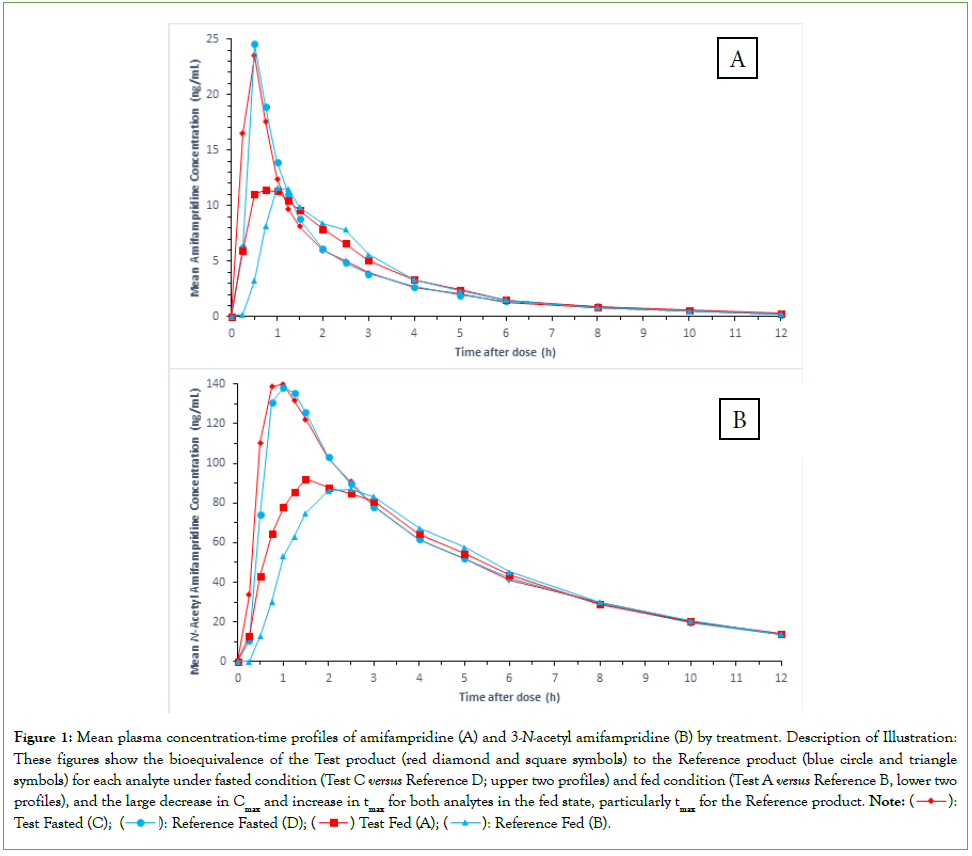
A summary of results using the 2-at-a-time principle for the comparative bioavailability of amifampridine and 3-N-acetyl amifampridine between the two products under fasted condition (Treatment C versus Treatment D) and fed condition (Treatment A versus Treatment B) is provided in Table 2 and Table 3, respectively, and associated fasted and fed mean plasma concentrations-time profiles are shown in Figures 1A and 1B, respectively. As the NAT2-status-by-treatment interaction was not statistically significant (p>0.05) for the Cmax and AUC parameters it was excluded in the final ANOVA model for the two analytes for both prandial conditions.
| Parameter, Unit | LSGM | LSGM Ratio C/D, % | 90% CI, % | Intra-Subject CV, % | |
|---|---|---|---|---|---|
| C, Fasted | D, Fasted | ||||
| Amifampridine | |||||
| Cmax, ng/mL | 18.8 | 19.7 | 95.1 | 82.9-109 | 23.9 |
| AUC0-t, h·ng/mL | 25.7 | 24.8 | 104 | 97.5-110 | 10.8 |
| AUC0-∞, h·ng/mL | 28.1 | 27.2 | 103 | 97.4-109 | 9.8 |
| 3-N-Acetyl Amifampridine | |||||
| Cmax, ng/mL | 143 | 144 | 99.2 | 93.7-105 | 9.8 |
| AUC0-t, h·ng/mL | 618 | 600 | 103 | 101-105 | 3.2 |
| AUC0-∞, h·ng/mL | 690 | 674 | 102 | 100-104 | 3.6 |
Note: n: Sample size; SA: Slow Acetylator; RIA: Rapid/Intermediate Acetylator; SD: Standard Deviation; MR: (Molar) Metabolic Ratio. a N=9 for Treatment A and N=8 for Treatment B, as one RIA individual had no evaluable AUC0-∞ data for Treatments A and B.
Table 2: Comparison of ln-transformed pharmacokinetic parameters of amifampridine and 3-N-acetyl metabolite for evaluation of the relative bioavailability of Test to Reference under fasted condition (Treatment C versus Treatment D) (N=18).
| Parameter, Unit | LSGM | LSGM Ratio A/B, % | 90% CI, % | Intra-Subject CV, % | |
|---|---|---|---|---|---|
| A, Fed | B, Fed | ||||
| Amifampridine | |||||
| Cmax, ng/mL | 8.34 | 8.26 | 101 | 85.2-120 | 30.1 |
| AUC0-t, h·ng/mL | 19.6 | 18.1 | 109 | 102-115 | 10.5 |
| AUC0-∞, h·ng/mLa | 22.6 | 21 | 108 | 102-114 | 9.6 |
| 3-N-Acetyl Amifampridine | |||||
| Cmax, ng/mL | 101 | 99 | 102 | 93.5-111 | 15.1 |
| AUC0-t, h·ng/mL | 536 | 512 | 105 | 101-108 | 5.6 |
| AUC0-∞, h·ng/mL | 608 | 580 | 105 | 101-109 | 6.2 |
Note: A: Test product amifampridine tablet 10 mg administered in the fed state; B: Reference product amifampridine phosphate tablet 10 mg base equivalent administered in the fed state; LSGM: Least-Squares Geometric Mean; CI: Confidence Interval; CV: Coefficient of Variation, N: Sample size. aN=17 as one individual had no evaluable AUC0-∞ data for Treatments A and B.
Table 3: Comparison of ln-transformed pharmacokinetic parameters of amifampridine and 3-N-acetyl metabolite for evaluation of the relative bioavailability of Test to Reference under fasted condition (Treatment C versus Treatment D) (N=18).
Under fasted and fed conditions, the 90% confidence intervals for the LSGM ratios of the Test to Reference treatments were within the standard equivalence range (80%, 125%) for all three pharmacokinetic parameters for amifampridine and 3-N-acetyl amifampridine. In the fed state, tmax was longer for the Reference product for both analytes (Figures 1A and 1B).
Figure 1: Mean plasma concentration-time profiles of amifampridine (A) and 3-N-acetyl amifampridine (B) by treatment. Description of Illustration: These figures show the bioequivalence of the Test product (red diamond and square symbols) to the Reference product (blue circle and triangle symbols) for each analyte under fasted condition (Test C versus Reference D; upper two profiles) and fed condition (Test A versus Reference B, lower two profiles), and the large decrease in Cmax and increase in tmax for both analytes in the fed state, particularly tmax for the Reference product. Note: : Test Fasted (C);
: Test Fasted (C);  : Reference Fasted (D);
: Reference Fasted (D);  Test Fed (A);
Test Fed (A);  : Reference Fed (B).
: Reference Fed (B).
Comparison of acetylator phenotype for amifampridine and 3-N-acetyl amifampridine
As the NAT2 status effect was statistically significant at the 5% significance level (p<0.05) for AUC and Cmax for treatment comparisons of both analytes using either the 2-at-a-time principle or combined treatments, a comparison of these pharmacokinetic parameters between the two phenotypes (slow NAT2 metabolizers versus rapid/ intermediate NAT2 metabolizers) pooled across the four treatments (A+B+C+D) is presented in (Table 4) for amifampridine and 3-N-acetyl amifampridine for the 18 evaluable individuals.
| Parameter, Unit | LSGM | LSGM Ratio SA/RIA, % | 90% CI, % | Inter-Subject CV, % | |
|---|---|---|---|---|---|
| SA | RIA | ||||
| Amifampridine | |||||
| Cmax, ng/mL | 29.6 | 5.39 | 550 | 432-701 | 68.2 |
| AUC0-t, h·ng/mL | 65.2 | 7.29 | 894 | 751-1065 | 46.7 |
| AUC0-∞, h·ng/mLa | 68.3 | 8.75 | 780 | 661-920 | 42.5 |
| t½z, h | 2.62 | 1.48 | 177 | 160-196 | 26.2 |
| 3-N-Acetyl Amifampridine | |||||
| Cmax, ng/mL | 99.2 | 145 | 68.6 | 61.8-76.2 | 27.2 |
| AUC0-t, h·ng/mL | 496 | 643 | 77.1 | 71.6-83.0 | 18.9 |
| AUC0-∞, h·ng/mL | 563 | 719 | 78.2 | 72.3-84.7 | 20.3 |
| t½z, h | 3.66 | 3.5 | 105 | 98.7-111 | 14.8 |
Note: LSGM: Least-Squares Geometric Mean; CI: Confidence Interval; CV: Coefficient of Variation; N: Sample size, SA: Slow Acetylator, RIA: Rapid/Intermediate Acetylator. aN=17 as one RIA individual had no evaluable AUC0-∞ data for Treatments A and B.
Table 4: Comparison of ln-transformed pharmacokinetic parameters of amifampridine and 3-N-acetyl metabolite for slow (SA) and Rapid/Intermediate (RIA) NAT2 metabolizers pooled across the four treatments (A+B+C+D) (N=18).
Based on Least-Squares Geometric Mean (LSGM) ratios, slow acetylators had statistically significant 5.5- to 8.9-fold higher amifampridine Cmax and AUC and a 1.8-fold longer t½z (1.48 to 2.62 hours), and 22%-31% lower 3-N-acetyl amifampridine Cmax and AUC, which resulted in a highly statistically significant NAT2 effect (p<0.0001) in the ANOVA for these parameters. LSGM metabolite t½z values were similar between the two phenotypes (Rapid/intermediate: 3.50 hours; Slow: 3.66 hours). In the slow acetylator group, the four subjects with only NAT2*5 variant alleles (*5A, *5B or *5C) tended to have the lowest amifampridine AUC values and the six subjects with at least one NAT2*6A variant allele (*6A/*6A, *5B/*6A or *6A/*7B) tended to have the highest amifampridine AUC values for the four treatments.
Mean plasma concentrations of 3-N-acetyl amifampridine were higher than mean amifampridine concentrations in all subjects at all time points for the four treatments. From data in Table 4, the resulting LSGM AUC and Cmax parameter values for the metabolite were 7.6- 8.2-fold and 3.3-fold higher than those for parent drug, respectively, for slow acetylators and 82-88-fold and 27-fold higher than those for parent drug, respectively, for rapid/intermediate acetylators pooled across the four treatments. Based on MR AUC0-t values (Table 1), molar concentrations of metabolite averaged 5.47- to 6.20-fold higher for slow acetylators under fasted and fed conditions. For rapid/intermediate acetylators, molar concentrations of metabolite averaged 56-57-fold in the fasted state and 86-91-fold in the fed state.
Food effect by acetylator status on pooled treatment data
The food-effect was assessed for each product separately (Test: A versus C; Reference: B versus D) using the 2-at-a-time principle and by pooling the four treatments in a single ANOVA for each analyte.
Pooled treatments: The lack of a differential effect of food on the two products (i.e., Test: A-C = Reference: B-D) was evaluated on pooled data from the four treatments using two statistical procedures as described in the Methods section. Both statistical methods produced the same treatment LSGM ratios for AUC and Cmax parameters, but the values for 90% confidence interval, intra-subject CV %, and p-values were different owing to the different values for the residual error and associated degrees of freedom for each ANOVA method. Regardless, the p-values that represent the product-by-food-effect interaction were >0.05 by both methods for the two analytes, thus justifying pooling the four treatments. For both analytes, there was less than a 7% difference in peak and total plasma exposures for the effect of food between the two products, and the 90% confidence intervals for the LSGM ratios of the Test (A-C) to Reference (B-D) treatments were within the standard equivalence range (80%, 125%) for the AUC parameters for amifampridine and 3-N-acetyl amifampridine and Cmax for metabolite. As there was no differential effect of food on the two products, the definitive food effect was evaluated on the pooled data across the four treatments ((A+B)/2 versus (C+D)/2) for AUC and Cmax for amifampridine and 3-N-acetyl amifampridine.
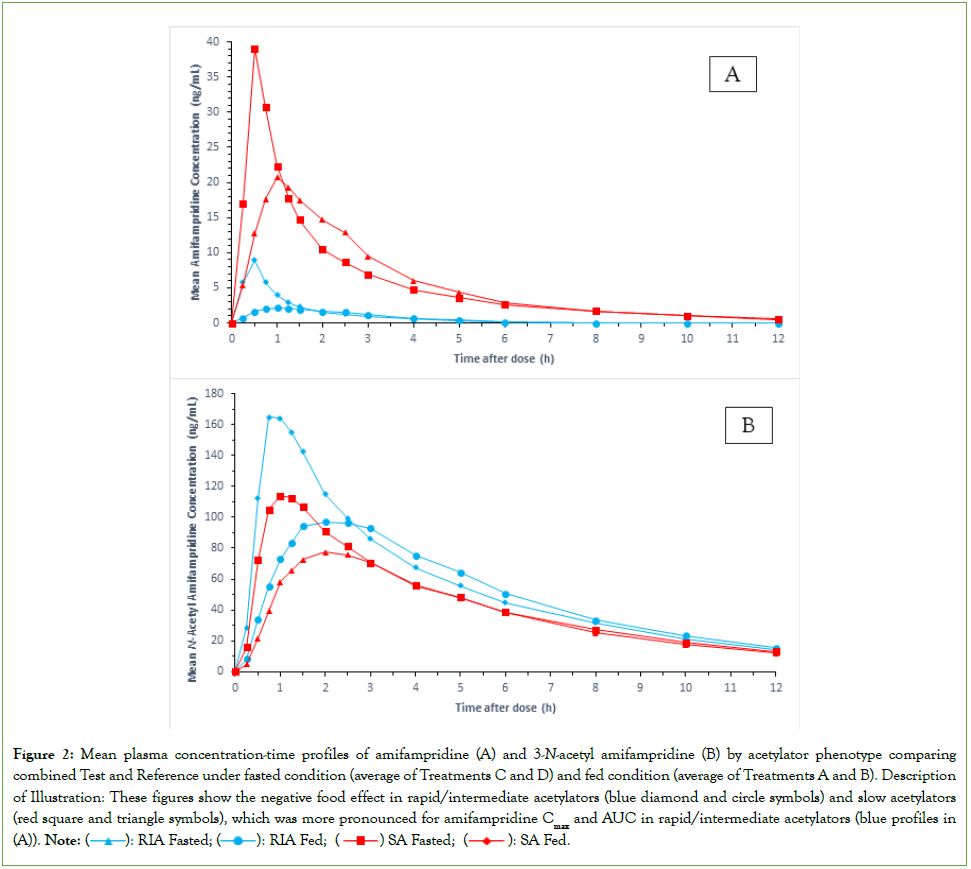
Food effect by acetylator status: Considering the NAT2-status-by- treatment interaction for the combined food effect was statistically significant (p≤0.001) for amifampridine AUC and Cmax parameters, the food effect results were separated by acetylator phenotype (N=9 per group) for this analyte (Table 5). As there was no statistically significant NAT2-status-by-treatment interaction for the combined food effect for the 3-N-acetyl amifampridine pharmacokinetic parameters (p>0.05), results are presented for the combined subjects (N=18) for the metabolite (Table 5). Mean plasma concentration-time profiles for amifampridine and 3-N-acetyl amifampridine pooled across the four treatments for the combined food effect are presented by acetylator phenotype in (Figures 2A and 2B), respectively.
Figure 2: Mean plasma concentration-time profiles of amifampridine (A) and 3-N-acetyl amifampridine (B) by acetylator phenotype comparing combined Test and Reference under fasted condition (average of Treatments C and D) and fed condition (average of Treatments A and B). Description of Illustration: These figures show the negative food effect in rapid/intermediate acetylators (blue diamond and circle symbols) and slow acetylators (red square and triangle symbols), which was more pronounced for amifampridine Cmax and AUC in rapid/intermediate acetylators (blue profiles in (A)).
| Parameter, Unit | LSGM | LSGM Ratio (A+B)/(C+D), % | 90% CI, % | Intra-Subject CV, % | p-valuea | |
|---|---|---|---|---|---|---|
| (A+B)/2, Fed | (C+D)/2, Fasted | |||||
| Amifampridine Slow NAT2 Metabolizers (N=9) | ||||||
| Cmax, ng/mL | 23.6 | 38.9 | 60.7 | 51.8-70.8 | 28 | 0.0006 |
| AUC0-t, h·ng/mL | 64.4 | 68.4 | 94.1 | 89.0-99.5 | 9.8 | < 0.0001 |
| AUC0-∞, h·ng/mL | 67.5 | 71.4 | 94.6 | 89.2-100 | 10.2 | 0.001 |
| Amifampridine Rapid/Intermediate NAT2 Metabolizers (N=9) | ||||||
| Cmax, ng/mL | 3.04 | 9.85 | 30.9 | 24.8-38.2 | 39.3 | 0.0006 |
| AUC0-t, h·ng/mL | 5.71 | 9.59 | 59.5 | 52.5-67.4 | 22.3 | < 0.0001 |
| AUC0-∞, h·ng/mL b | 7.12 | 10.8 | 66.2 | 57.9-75.6 | 22.1 | 0.001 |
| 3-N-Acetyl Amifampridine (N=18) | ||||||
| Cmax, ng/mL | 100 | 143 | 69.7 | 65.8-73.8 | 14.6 | 0.6465 |
| AUC0-t, h·ng/mL | 524 | 609 | 86 | 84.3-87.7 | 4.9 | 0.1597 |
| AUC0-∞, h·ng/mL | 594 | 682 | 87.1 | 85.2-89.1 | 5.7 | 0.1156 |
Note: LSGM: Least-Squares Geometric Mean; CI: Confidence Interval; CV: Coefficient of Variation; N: Sample size. ap-value for NAT2-status-by-treatment interaction in full model (N=18). Non-significant interaction was excluded in ANOVA model for 3-N-acetyl amifampridine; bN=8 as one individual had no evaluable AUC0-∞ data for Treatments A and B.
Table 5: Comparison of ln-transformed pharmacokinetic parameters of amifampridine and 3-N-acetyl metabolite for evaluation of food effect on the combined products (Fed: Treatments A+B versus fasted: Treatments C+D)
Inclusion of the statistically significant NAT2-status-by-treatment term in the final ANOVA model for the three food-effect comparisons (A versus C and B versus D using the 2-at-a-time principle and (A+B)/2 versus (C+D)/2 using the combined treatments) for amifampridine AUC and Cmax parameters did not bias the treatment effect because the study was balanced for NAT2 acetylator status across the 18 evaluable subjects (9 rapid/intermediate and 9 slow acetylators, and 9 subjects per sequence) with maximal balance in each sequence (5 slow and 4 rapid/intermediate in sequence ABCD and 5 rapid/intermediate and 4 slow in sequence BADC). Therefore, the LSGM ratios were the same with or without inclusion of the interaction term in the ANOVA for the parameters (AUC0-t and Cmax) that maintained this balance and had complete data for the 18 subjects.
Results for the combined products showed that the high-fat meal decreased peak and total plasma exposures of amifampridine and metabolite but the effect was more pronounced on amifampridine for rapid/intermediate acetylators. In that group, amifampridine AUC values decreased 34%-40% based on LSGM fed/fasted ratios of 60%- 66%, and Cmax decreased 69% as evidenced by a LSGM fed/fasted ratio of 31%; the 90 confidence intervals for all AUC and Cmax LSGM fed/fasted ratios were outside the standard equivalence interval of 80%-125% (Table 5). For slow acetylators, AUC was unaffected by food based on LSGM fed/fasted ratios of 94%-95% and associated 90% confidence intervals within the standard equivalence range. However, food significantly decreased amifampridine Cmax by 39% in slow acetylators as evidenced by a LSGM fed/fasted ratio of 61% and an associated 90% confidence interval entirely outside the standard equivalence interval (80%-125%). For the food-effect comparisons of these amifampridine Cmax and AUC parameters, the post-hoc study power was >80% at the 5% significance level (1-sided) to detect at least a 25% decrease in AUC (observed 34%-40%) and 40% decrease in Cmax (observed 69%) for rapid/intermediate acetylators, and at least a 15% decrease in AUC (observed 5%-6%) and 30% decrease in Cmax (observed 39%) for slow acetylators. The negative food effect was less apparent for 3-N-acetyl amifampridine, with LSGM fed/fasted ratios of 70% for Cmax and 86%-87% for AUC parameters of the combined acetylator groups.
Relative to the fasted state the high-fat meal increased amifampridine median tmax in the two acetylator groups from 0.5 hours to 0.75-1 hours for the Test product (Treatment C to Treatment A) and from 0.5 hours to 1.15-1.53 hours for the Reference product (Treatment D to Treatment B). Similar increases in median tmax were observed for metabolite in the two acetylator groups by product. The increase in tmax for both analytes in the fed state was the greatest for rapid/ intermediate acetylators administered Reference product, with median tmax prolonged by 1.03 hours for amifampridine and 1.75 hours for metabolite (Table 1). Mean t½z was similar in the fasted and fed states within each acetylator group (2.49-2.82 hours and 1.33-1.63 hours for amifampridine slow and rapid/intermediate acetylators, respectively, and 3.40-3.83 hours for metabolite). The molar MR AUC0-t was about 10-fold larger in rapid/intermediate acetylators than in slow acetylators in the absence of food (Table 1, Treatments C and D); the ratio increased further by approximately 1.6-fold in rapid/intermediate acetylators following a high-fat meal (from 56-57 to 86-91), as a consequence of a larger decrease in parent AUC, whereas it was minimally affected by food in slow acetylators (Table 1, Treatments A and B).
Safety and tolerability
The overall incidence of Treatment-Emergent Adverse Events (TEAEs) was 9 reported in 7 subjects (4 males and 3 females, 3 rapid/ intermediate and 4 slow acetylators). One participant was discontinued because of an adverse event (asymptomatic COVID-19). Of the 5 TEAEs that were probably related to the investigational product, 4 occured in slow acetylators under fasted condition (2 incidences of paresthesia (oral and fingers) in one participant receiving Test, lower abdominal pain in one participant receiving Reference, and throbbing headache in another participant receiving Reference), and 1 occurred in a rapid/intermediate acetylator receiving Test-fasted (trunk rash). No significant TEAEs were reported and all TEAEs were either mild or moderate in severity with most resolved by completion of the clinical phase of the study. There was no notable difference in the incidence or nature of TEAEs between formulations or prandial status.
Discussion
Peak and total plasma exposures of amifampridine and its 3-N-acetyl metabolite were equivalent between Ruzurgi and Firdapse® following a single 10-mg dose in either the fasted state (Treatment C versus Treatment D) or fed state (Treatment A versus Treatment B). Therefore, dosing regimens of the two products can be considered interchangeable in the fasted and fed states.
Slow acetylators had significantly higher plasma concentrations of amifampridine and lower plasma concentrations of 3-N-acetyl- amifampridine for both products. The terminal disposition half-life of amifampridine was significantly longer in slow acetylators and unaffected by acetylator phenotype for 3-N-acetyl amifampridine. These findings are similar to those reported in the studies by Haroldsen, et al., [3,7].
A high-fat meal decreased peak and total plasma exposures of amifampridine and 3-N-acetyl amifampridine, consistent with the findings of Haroldsen, et al., [8]. However, the effect was more pronounced on amifampridine for rapid/intermediate acetylators, indicating the importance of knowing an individual’s acetylator status to avoid potential underdosing either product with a high-fat meal. Our results support the proposal by Garovoy, et al., that fast acetylators should take 3-4-diaminopyridine without food [1].
The negative food effect and the significant NAT2-status by food- effect interaction for which rapid/intermediate NAT2 acetylators showed larger percentage decreases in plasma concentrations of amifampridine relative to slow NAT2 acetylators can be rationalized based on the pharmacokinetic properties of amifampridine, notably the changes in molar MR AUC0-t, and the characteristics of the NAT2 enzyme distribution in the small intestine in rapid and slow acetylators.
As amifampridine systemic clearance is characterized mainly by high metabolite formation clearance to a single metabolite (3-N-acetyl amifampridine) and recovery of parent and metabolite in urine is near complete at 93%-100% under fasted and fed conditions [8], any effect of food intake on amifampridine pharmacokinetics is expected to alter metabolite formation either systemically or during first-pass metabolism in the gastrointestinal tract and liver, the latter of which influences drug bioavailability. The approximate 1.6-fold increase in molar MR AUC0-t following a high-fat meal in rapid/intermediate acetylator phenotypes indicates an increase in apparent formation clearance of amifampridine to 3-N-acetyl amifampridine assuming no change in metabolite clearance. The higher apparent formation clearance to metabolite and larger decrease in parent than metabolite AUC and Cmax in rapid/ intermediate acetylator following consumption of a high-fat meal is a reflection of increased systemic metabolite formation, decreased bioavailability from increased metabolite formation during first-pass intestinal metabolism, and/or a disproportionate larger increase in systemic metabolite formation than in pre-systemic hepatic drug bioavailability. These possible mechanisms are discussed below.
Certain high clearance drugs, like propranolol, exhibit significant positive food effects (increase in oral AUC) because food increases splanchnic hepatic blood flow [9], which in turn increases hepatic drug bioavailability and systemic drug clearance, but disproportionately higher for bioavailability [10,11]. Amifampridine is considered a high hepatically cleared drug [3,7], clearance being flow dependent, and notably more in rapid than slow acetylators. Therefore, plasma clearance and bioavailability for rapid acetylators is expected to be more affected by possible increase in liver blood flow from post-prandial effects, whereby any increased hepatic blood flow, if it occurs, would be restricted to early times when food is digested and absorbed and drug absorption occurs, such that transient increase in drug bioavailability with concomitant decrease in metabolite formation during hepatic first-pass metabolism would occur at the early post-dose times and elimination of absorbed parent drug via formation to its 3-N-acetyl metabolite would occur predominantly thereafter. That a negative not a positive food effect was observed for amifampridine in our study suggests there may be a disproportionate larger increase in systemic metabolite formation than in pre-systemic hepatic drug bioavailability for this high hepatically cleared drug, or other mechanisms may also be contributing.
Conversely, certain low clearance drugs, like isoniazid, show negative food effects [12,13], in agreement with a statistical model that predicted for compounds that are completely absorbed, only those that are highly soluble and highly hydrophilic are prone to show negative food effects [14]. Isoniazid and amifampridine are pyridine-based drugs that are highly metabolized by NAT2, highly soluble, highly hydrophilic, and completely absorbed orally, but isoniazid is a high bioavailable, low hepatically cleared drug [12]. These characteristics suggest that the decrease in amifampridine AUC could also result from contributions from increase in intestinal luminal NAT2 metabolism and not exclusively from a disproportionate higher increase in systemic clearance than pre- systemic hepatic bioavailability induced by increase in liver blood flow, as an increase in liver blood flow is predicted to have no effect on these parameters for low hepatically extracted drugs like isoniazid following food intake [9].
NAT has been identified as a polymorphic enzyme whose pharmacokinetic activity follows Michaelis-Menten (saturable) kinetics with acetylation of various drugs including isoniazid [4], and the pharmacokinetics of amifampridine implicate a saturable first-pass metabolism with increasing single oral dose [3]. The distribution of NAT2 activity along the length of the small intestine decreased about 2-fold from the duodenum to the ileocecal junction in two rapid acetylators but remained lower and invariant in two slow acetylators [15], suggesting intestinal NAT2 activity is regional site specific in rapid acetylators. Therefore, there is the possibility that higher concentrations in the fasted state for highly soluble, highly permeable drugs like isoniazid and amifampridine may lead to saturation of intestinal NAT2, whereas intestinal concentrations are lower in the fed state owing to higher intestinal fluid volumes and slower entry of drug from stomach to small intestine (longer tmax) promoting increased enteric metabolism upon entry to the proximal region of the duodenum where NAT2 activity is highest in rapid/intermediate acetylators. Increased residence time in the upper duodenum and prolonged intestinal transit time from the high-fat meal would also promote more efficient intestinal metabolism in rapid/intermediate acetylators [16]. The interplay of pre-systemic enteric and hepatic NAT2 metabolism determines the oral bioavailability. Therefore, food intake may alter the interplay of pre-systemic enteric and hepatic NAT2 metabolism and post-systemic formation clearance to metabolite by enhancing enteric metabolism, reducing pre-systemic hepatic metabolism and increasing post-systemic hepatic metabolism with a resultant overall greater decrease in AUC and Cmax of parent than of metabolite.
The effect of food was less for metabolite AUC in rapid/intermediate acetylators because 3-N-acetyl amifampridine is the sole formed metabolite and constitutes at least 74% of administered dose with or without food [8]. Therefore, the counterbalancing effects of pre-and post-systemic metabolism produced minimal percentage decrease in metabolite AUC in the presence of food, considering the high percentage of administered dose that is converted to metabolite. Also, as the hydrophilicity of amifampridine is minimally affected by the addition of an acetyl group, the decrease in Cmax of both metabolite and parent may be explained by a decrease in the rate of permeability during intestinal absorption of parent drug and enteral formed metabolite in the presence of food.
Overall, based on the pharmacokinetic characteristics of amifampridine and its 3-N-acetyl metabolite, each subject’s assigned acetylator phenotype was consistent with the activity of their NAT2 alleles from the genotype test. That is, individuals with two low- activity NAT2 alleles were classified as a slow acetylator phenotype, whereas those with one or two high-activity *4 alleles were classified as intermediate and rapid acetylator phenotypes, respectively.
Compared to rapid/intermediate acetylators, slow acetylators had more TEAEs that were probably related to the investigational product, and all occurred under fasted condition, which is the population and prandial condition that had the highest plasma concentrations of amifampridine.
Conclusion
Comparative bioavailability under fasted and fed conditions
Peak and total plasma exposures of amifampridine and its 3-N-acetyl metabolite were equivalent between Ruzurgi® (amifampridine) tablet 10 mg and Firdapse® (amifampridine phosphate) tablet 10 mg (base equivalent) following a single-dose administration in either the fasted or fed state.
Food effect by acetylator status
A high-fat meal decreased peak and total plasma exposures and increased time to reach maximum plasma concentrations of amifampridine and 3-N-acetyl amifampridine, but the effect on exposures was more pronounced on amifampridine for rapid/intermediate acetylators. The negative food effect was similar between the two products and less apparent for the metabolite, though tmax of both analytes was more prolonged for rapid/intermediate acetylators administered Reference product in the fed state. The rate but not the extent of amifampridine absorption was decreased in slow acetylators, whereas both rate and extent of absorption were reduced in rapid/intermediate acetylators relative to the fasted state for the combined data from both products. The decreased plasma exposures of amifampridine likely resulted from changes in post-systemic and/or pre-systemic NAT2 metabolism.
Safety and tolerability
A single oral dose of 10 mg of the Test and Reference products was well tolerated under fasting and fed conditions. There was no notable difference in the incidence or nature of TEAEs between formulations or prandial status, though slow acetylators had more TEAEs related to the investigational products.
Clinical implications
Ruzurgi® and Firdapse® were demonstrated to be bioequivalent under fasted and fed conditions, and thus dosing regimens of the two products can be considered interchangeable. Our study is intended to make prescribers aware of the differential effect of acetylator status on the food effect, and, if necessary, guide their decision in making dose adjustments.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Statement
The study and manuscript preparation were funded by Catalyst Pharmaceuticals, Inc., Coral Gables, Florida USA.
Author Contributions
Author contributions were as follows: study conceptualization, design, data interpretation, and initial draft of manuscript, Dr. Gallicano; bioanalytical method development and validation, Dr. Khadang; oversight of clinical trial operations and conduct, Dr. Boldingh; and study design, Dr. Ingenito. All authors contributed to review and writing of the manuscript and gave final approval to the manuscript.
Declaration of Competing Interest
Dr. K. Gallicano is a consultant for AXIS Clinicals, Dr. P. Boldingh and Dr. Khadang are employees of AXIS Clinicals, and Dr. G. Ingenito is an employee of Catalyst Pharmaceuticals, Inc. The study was sponsored and the manuscript preparation was funded by Catalyst Pharmaceuticals, Inc.
References
- Garovoy MR, Haroldsen PE, Musson DG. U.S. patent application for methods of administering 3,4-diaminopyridine. U.S. patent no. 11,845,977 B2. Dec. 19, 2023.
- Sim E, Abuhammad A, Ryan A. Arylamine N‐acetyltransferases: From drug metabolism and pharmacogenetics to drug discovery. Br J Pharmacol. 2014;171(11):2705-2725.
[Crossref] [Google Scholar] [PubMed]
- Haroldsen PE, Garovoy MR, Musson DG, Zhou H, Tsuruda L, Hanson B, et al. Genetic variation in aryl N‐acetyltransferase results in significant differences in the pharmacokinetic and safety profiles of amifampridine (3,4‐diaminopyridine) phosphate. Pharmacol Res Perspect. 2015;3(1):e00099.
[Crossref] [Google Scholar] [PubMed]
- Fukunaga K, Kato K, Okusaka T, Saito T, Ikeda M, Yoshida T, et al. Functional characterization of the effects of N-acetyltransferase 2 alleles on N-acetylation of eight drugs and worldwide distribution of substrate-specific diversity. Front Genet. 2021;12:652704.
[Crossref] [Google Scholar] [PubMed]
- Sabbagh A, Darlu P, Crouau-Roy B, Poloni ES. Arylamine N-acetyltransferase 2 (NAT2) genetic diversity and traditional subsistence: a worldwide population survey. PloS one. 2011;6(4):18507.
[Crossref] [Google Scholar] [PubMed]
- Meyer UA, Zanger UM. Molecular mechanisms of genetic polymorphisms of drug metabolism. Annu Rev Pharmacol Toxicol. 1997;37(1):269-296.
[Crossref] [Google Scholar] [PubMed]
- Haroldsen PE, Sisic Z, Datt J, Musson DG, Ingenito G. Acetylator status impacts amifampridine phosphate (Firdapse™) pharmacokinetics and exposure to a greater extent than renal function. Clin Ther. 2017;39(7):1360-1370.
[Crossref] [Google Scholar] [PubMed]
- Haroldsen PE, Musson DG, Hanson B, Quartel A, O’Neill CA. Effects of food intake on the relative bioavailability of amifampridine phosphate salt in healthy adults. Clin Ther. 2015;37(7):1555-1563.
[Crossref] [Google Scholar] [PubMed]
- Marasanapalle VP, Boinpally RR, Zhu H, Grill A, Tang F. Correlation between the systemic clearance of drugs and their food effects in humans. Drug Dev Ind Pharm. 2011;37(11):1311-1317.
[Crossref] [Google Scholar] [PubMed]
- Olanoff LS, Walle T, Cowart TD, Walle UK, Oexmann MJ, Conradi EC. Food effects on propranolol systemic and oral clearance: Support for a blood flow hypothesis. Clin Pharmacol Ther. 1986;40(4):408-414.
[Crossref] [Google Scholar] [PubMed]
- Walle T, Fagan TC, Walle UK, Oexmann MJ, Conradi EC, Gaffney TE. Food‐induced increase in propranolol bioavailability-Relationship to protein and effects on metabolites. Clin Pharmacol Ther. 1981;30(6):790-795.
[Crossref] [Google Scholar] [PubMed]
- Saktiawati AM, Sturkenboom MG, Stienstra Y, Subronto YW, Sumardi, Kosterink JG, et al. Impact of food on the pharmacokinetics of first-line anti-TB drugs in treatment-naive TB patients: A randomized cross-over trial. J Antimicrob Chemother. 2016;71(3):703-710.
[Crossref] [Google Scholar] [PubMed]
- Lin HC, Yu MC, Liu HJ, Bai KJ. Impact of food intake on the pharmacokinetics of first-line antituberculosis drugs in Taiwanese tuberculosis patients. J Formos Med Assoc. 2014;113(5):291-297.
[Crossref] [Google Scholar] [PubMed]
- Gu CH, Li H, Levons J, Lentz K, Gandhi RB, Raghavan K, et al. Predicting effect of food on extent of drug absorption based on physicochemical properties. Pharm Res. 2007;24:1118-1130.
[Crossref] [Google Scholar] [PubMed]
- Hickman D, Pope J, Patil SD, Fakis G, Smelt V, Stanley LA, et al. Expression of arylamine N-acetyltransferase in human intestine. Gut. 1998;42(3):402-409.
[Crossref] [Google Scholar] [PubMed]
- Briguglio M, Hrelia S, Malaguti M, Serpe L, Canaparo R, Dell’Osso B, et al. Food bioactive compounds and their interference in drug pharmacokinetic/pharmacodynamic profiles. Pharmaceutics. 2018;10(4):277.
[Crossref] [Google Scholar] [PubMed]
Citation: Gallicano K, Ingenito G, Khadang A, Boldingh P (2024) Comparative Bioavailability of Two Tablet Formulations of Amifampridine with and without Food, and the Impact of Acetylator Status on the Pharmacokinetic Food Effect. J Bioequiv Availab. 16:566.
Copyright: © 2024 Gallicano K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.