Indexed In
- The Global Impact Factor (GIF)
- CiteFactor
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- Virtual Library of Biology (vifabio)
- International committee of medical journals editors (ICMJE)
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 23, Issue 3
Comparative Assessment of Two Dentin Desensitizers for Dentin Tubule Occlusion and Cytological Safety
Qotoof Kefah Alsenan1*, Deepak Mehta2, Zahi Badran3, Betul Rahman3 and Soumya Aravind42Department of Cariology, SIMATS Deemed University, Chennai, India
3Department of Periodontology, University of Sharjah,Sharjah, UAE
4Department of Dental Biomaterials, University of Sharjah, Sharjah, UAE
Received: 28-Apr-2023, Manuscript No. OHDM-23-21160; Editor assigned: 01-May-2023, Pre QC No. OHDM-23-21160 (PQ); Reviewed: 15-May-2023, QC No. OHDM-23-21160; Revised: 13-Sep-2024, Manuscript No. OHDM-23-21160 (R); Published: 30-Sep-2024, DOI: 10.35248/2247-2452.24.23.1121
Abstract
Objectives: The objectives of this in vitro study were to compare the occlusion profile and gingival safety of Predicta™ Bioactive Desensitizer (PBD, parkell, Edgewood, NY, USA), a methacrylate-free, nanohydroxyapatite-based desensitizer with Gluma® desensitizer liquid (GLU, Heraeus Kulzer, Germany).
Materials and methods: In this study, occlusal surfaces were prepared from 30 caries-free human molars and were randomly allocated to three groups each consisting of 10 samples. Samples in group A (n=10), group B (n=10) were treated with predicta™ bioactive desensitizer, gluma® desensitizer liquid. Group C (n=10) was the control group and samples in this group were left immersed in artificial saliva. Micromorphological analysis using scanning electron microscope was performed to assess tubular occlusion. A blinded reviewers independently scored the level of tubule occlusion as type 0 (no occlusion; score 0), type 1 (partial occlusion; ≤ 25%; score 1), type 2 (partial occlusion; 25-75%; score 2), type 3 (complete occlusion; >75%; score 3). Furthermore, the gingival safety of predicta™ bioactive desensitizer and gluma® desensitizer liquid was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (XTT) test. Means were compared using One-way Analysis of Variance (ANOVA). Post-hoc comparisons were made using Tukey test. A 2-sided test with p<0.05 was considered to be statistically significant.
Results: The mean occlusion scores computed from micromorphological analysis using scanning electron microscope in group A (predicta; n=10) and group B (gluma; n=10) were 1.6 ± 1.07 (p<0.001 vs. control) and 2.3 ±1.05 (p<0.001 vs. control), respectively. Results of the XTT test indicated that the percentage of cell viability with varying concentrations of predicta bioactive desensitizer at 1, 24 and 48 hours remained significantly higher as compared to gluma® desensitizer liquid (p<0.001) at 1 hour, 24 hours and 48 hours after treatment.
Conclusions: The occlusive efficacy of predicta bioactive desensitizer seems to be comparable to that of gluma desensitizer liquid. On the other hand, predicta bioactive desensitizer may be safer to the gingival milieu.
Keywords
Dentinal hypersensitivity; Dentin desensitization; Gluma desensitizer liquid; Predicta™ bioactive desensitizer
Introduction
Dentin hypersensitivity is a relatively common presentation in “real-world” clinical settings with a predilection for female gender and people aged above 30-40 years [1,2]. While prevalence studies have yielded heterogenous results, the best estimated prevalence could be about 11.5% [3]. The nociceptive consequences arising from the “exposed dentin” adversely impact several indices of Health-Related Quality of Life (HRQoL) [4,5]. Drawing premise from brannstrom’s hydrodynamic theory, several physical, chemical and nerve desensitizing options have become available for treating dentin hypersensitivity [6]. However, none of these extant options are “gold-standards” and the choice of treatment depends on an evidence based balance of safety efficacy considerations and contextual factors presented by individual patients and their preferences for clinic-based or home-based treatments. Among these options, bioactive dentin desensitizers have attained a conspicuous prominence. An editorial by Vallittu, et al. explains that the terms “bioactive” and “biomineralization” must be reserved for evidence based materials, with optimal ion-release and biomineralization characteristics [7]. Previous studies have indicated that the Predicta™ Bioactive Desensitizer (PBD, Parkell, Edgewood, NY, USA), a methacrylate-free, nanohydroxyapatite based desensitizer provides for immediate tubular occlusion and continued calcium and phosphate ion remineralization for further occlusion. Extending on these previous studies, we conducted an in vitro study to compare the occlusion profiles and gingival safety of predicta™ bioactive desensitizer with Gluma® Desensitizer liquid (GLU, Heraeus Kulzer, Germany), an established occlusive resinous agent, which contains glutaraldehyde (5%) and 2-Hydroxyethyl-Methacrylate (HEMA). Results of this comparative study are presented herein [8].
Materials and Methods
Preparation of samples
Recently 30 extracted non-carious human molars were collected from the oral surgery clinic of college of dental medicine, university of Sharjah, after obtaining informed consent from patients (Approval code REC-22-04-06-S). The research ethics committee of the institution has given their approval for this in vitro study [9].
Teeth were subsequently washed with distilled water and stored in normal saline (0.9% w/v) at room temperature.
The crown sectioned from the roots and then dentin disc were prepared using precision cutting machine (Isomet®1000 precision sectioning saw-Buehler, USA). Specimens were prepared perpendicular to the long axis of the tooth from the dentin section closest to the pulp chamber away from the pulp horn and enamel. The occlusal surfaces were then flattened with a polishing machine (Metkon® FORCIPOL 2v grinder and polisher, Turkey) to obtain 2 mm-thick dentin slices. Then the occlusal side of each slice was polished for 30 seconds with 600-grit paper (3 M, St. Paul, MN, USA) in a circular motion under running water to establish a standardized smear layer.
Subsequently, all the samples were kept in 17% Ethylenediaminetetraacetic Acid (EDTA) for 40 minutes to completely open the dentinal tubules and then in distilled water for 12 minutes to remove residual smear layers [10-14]. All samples were then dehydrated in a graded series of ethanol (10% to 90%) for 30 minutes each. These samples were preserved in artificial saliva.
The samples were divided randomly into three groups, each with 10 specimens, and treated as follow:
• Group A: Samples treated with predicta bioactive desensitizer.
• Group B: Samples treated with gluma desensitizer liquid.
• Group C: Control group.
The two desensitizers evaluated in the study were applied to the samples in conformity to the instructions from respective manufacturers.
As per manufacturer’s specifications, samples in group a were rinsed with warm water, dried with absorbent cotton and a coat of Predicta™ Bioactive Desensitizer (PBD, parkell, Edgewood, NY, USA )was applied and rubbed gently for 10 to 20 seconds. Excess product was removed by wiping the samples with a cotton pledget and were air-dried using an air blower [15].
Gluma desensitizer liquid (gluma desensitizer, Heraeus Kulzer, Hanau, Germany) was applied to water-washed and air-dried samples in group B. After leaving this sample undisturbed for about a minute, all the samples were air-dried to remove fluid film and shininess.
Analysis
Observations for the samples were performed under different magnifications 5,000 and 10,000X, using a Scanning Electron Microscope (SEM) (VEGA3 XM, TESCAN, Brno, Czech Republic) operating at 20 kV acceleration voltage and 15 mm working distance.
Micro-morphological analysis was done after sputter coating with 100 Å gold-palladium (EMS 7620 mini sputter coater, Hatfield, PA) in order to create a conductive specimen surface and reduce electron charging, which may reduce the quality of the image.
The specimens were analyzed by a three blinded reviewers then independently assessed the images to score the level of tubule occlusion as type 0 (no occlusion; score 0), type 1 (partial occlusion; ≤ 25%; score 1), type 2 (partial occlusion; 25-75%; score 2), type 3 (complete occlusion; >75%; score 3).
Cell culture methodology
Human Gingival Fibroblasts (HGF) cell culture: HGF cells (Cryovial: 300703, procured from CLS Cell Lines Service GmbH, Dr. Eckener-Strabe 8, 69214 Eppelheim, Germany) were revived in DMEM/F-12 cell culture medium (Sigma-Aldrich, USA) supplemented with 10% Fetal Bovine Serum (FBS, Sigma- Aldrich, USA) and 1% penicillin-streptomycin (Sigma-Aldrich, USA). The cells were maintained at 37℃ in a CO2 incubator with 5% CO2 and 95% air [16].
Cell viability assay: The cytotoxic effects of gluma desensitizer liquid and predicta™ bioactive desensitizer on HGF cells were evaluated using the XTT assay (XTT proliferation kit, Roche, Sigma-Aldrich). HGF cells were seeded onto 96-well plates at a density of 10 cells/well in a final volume of 100 μL of complete DMEM/F-12 cell culture medium. After 24 h, the cultured HGFs were treated with gluma desensitizer liquid and predicta™ bioactive desensitizer with varying concentrations (0.1. 0.3, 0.5 and 1%) for 1, 24 and 48 h. After the respective incubation, XTT assay was carried out following the manufacturer’s instruction [17]. Around 50 μL of the XTT reagent was added to each well and the plates were kept for 4 h incubation. The absorbance was then measured using a plate reader (synergy H1 microplate reader, biotek instruments, USA) at 450 and 630 nm wavelengths. Cells grown in the culture medium was considered as the positive control and the once treated with 10% DMSO was taken as the negative control. Cell viability was calculated as the percentage with respect to the positive control group, and the results were analyzed with a one-way analysis of variance followed by Tukey’s post hoc tests. Assessments of cell viability was also made by checking the cell morphology in a phase contrast microscopy after the respective treatments at 1, 24 and 48 hours.
Statistical analysis
Data was tabulated with computations of mean for central tendencies and standard deviation for measures of dispersion. Means were compared using One-way Analysis of Variance (ANOVA). Post-hoc comparisons were made using Tukey test. A 2-sided test with a 95% confidence level P<0.05 was considered to be statistically significant. All the statistical tests were performed with the IBM Statistical Package for the Social Sciences (SPSS) Statistics for Windows, version 27.0, Armonk, NY, USA.
Results
Figure 1 shows the representative scanning electron microscope images for tubular occlusion in group
• Predicta™ bioactive desensitizer, group.
• Gluma desensitizer liquid and group.
• Control at two different magnifications 5,000X and 10,000X.
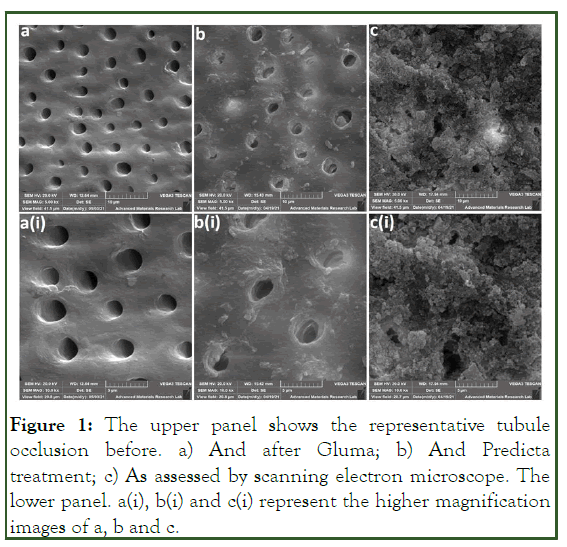
Figure 1: The upper panel shows the representative tubule occlusion before. a) And after Gluma; b) And Predicta treatment; c) As assessed by scanning electron microscope. The lower panel. a(i), b(i) and c(i) represent the higher magnification images of a, b and c.
Representative scanning electron microscope images of our study groups showed almost a complete tubular occlusion in group A and partial tubular occlusion in group B1 compared to the control group samples that demonstrate on total open dentinal tubules [18].
Table 1 depicts the mean difference of post-treatment tubular occlusion scores between groups based on the blinded reviewer’s scores.
| Inter-group comparisons | Mean difference (i-ii)* |
P-value** | |
|---|---|---|---|
| i | ii | ||
| Predicta™ bioactive desensitizer | Gluma desensitizer liquid | 0.7 | 0.18 |
| Predicta™ bioactive desensitizer | Control | 2.3 | <0.001 |
| Gluma desensitizer liquid | Control | 1.6 | <0.001 |
*Computed using ANOVA and Tukey adjustment,**p<0.001.
Table 1: Mean difference of post-treatment tubule occlusion score between groups.
The mean occlusion scores in group.
• Predicta™ bioactive desensitizer and group.
• Gluma desensitizer liquid were 2.3 ± 1.05 (p<0.001 control)
and 1.6 ± 1.07 (p<0.001 control), respectively. Significant differences were detected between group A, B with group C
(p>0.001). However, the blinded reviewer evaluated scores did
not reveal any significant differences between group predicta™
bioactive desensitizer and gluma desensitizer liquid groups.
Cell culture results
The results of the cell viability assay Figure 2 showed that for the gluma desensitizer, the gingival fibroblasts were viable only in the lowest concentration which is 0.10% in all time points [19]. During the initial 1 h, the cells cultured on all the concentrations were viable. However at 24 h and 48 h, only the cells treated with 0.1% gluma was showing the viability. The rest three concentrations were found to be cytotoxic in nature. For the 0.1%, a decrease in cell viability was observed after 48 h of culture.
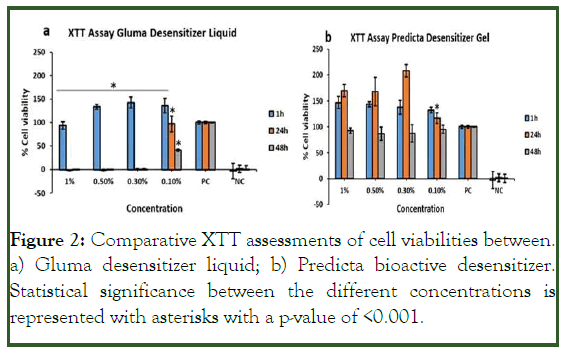
Figure 2: Comparative XTT assessments of cell viabilities between. a) Gluma desensitizer liquid; b) Predicta bioactive desensitizer. Statistical significance between the different concentrations is represented with asterisks with a p-value of <0.001.
Compared to gluma, the predicta bioactive desensitizer Figure 2b at all concentrations in all time points were non-toxic to the gingival fibroblasts. An increase in the cell viability was observed at 24 h for the 1%, 0.5% and 0.3% compared to the positive control cells. However, no statistical significance in cell viability was observed for all the concentrations of predicta after 48 h of incubation.
The cytotoxic effect of the gluma desensitizer was also evident from the morphological analysis of the HGF cells (Figures 3 and 4). The cells demonstrated a round morphology in the 1%, 0.5% and 0.3% concentrations at 24 h and 48 h compared to the typical elongated morphology of the gingival fibroblasts (Figure 5). However, those cells grown in 0.1% showed the elongated morphology at 24 h of culture [20]. A decrease in cell density followed by the appearance of round cells were observed in the 0.1% gluma at 48 h. The morphological analysis of the HGF cultured in the predicta desensitizer Figure 4 at all concentrations in all time points showed the characteristics elongated morphology of the gingival fibroblasts proving the cytofriendly nature of the predicta over gluma (Figure 6).
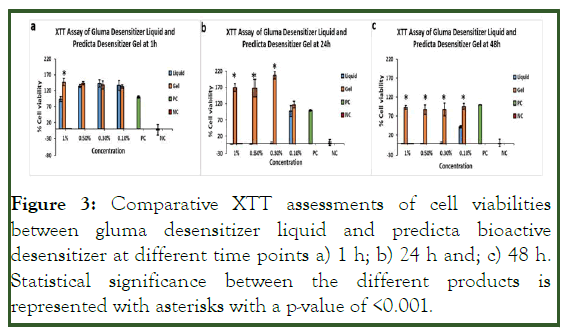
Figure 3: Comparative XTT assessments of cell viabilities between gluma desensitizer liquid and predicta bioactive desensitizer at different time points a) 1 h; b) 24 h and; c) 48 h. Statistical significance between the different products is represented with asterisks with a p-value of <0.001.
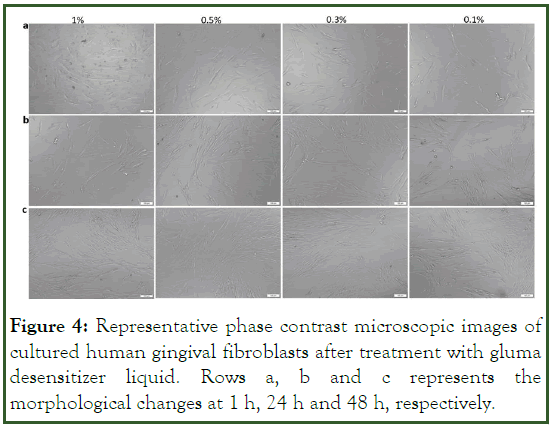
Figure 4: Representative phase contrast microscopic images of cultured human gingival fibroblasts after treatment with gluma desensitizer liquid. Rows a, b and c represents the morphological changes at 1 h, 24 h and 48 h, respectively.
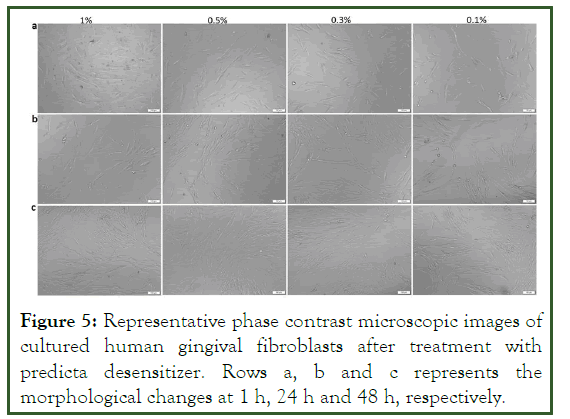
Figure 5: Representative phase contrast microscopic images of cultured human gingival fibroblasts after treatment with predicta desensitizer. Rows a, b and c represents the morphological changes at 1 h, 24 h and 48 h, respectively.
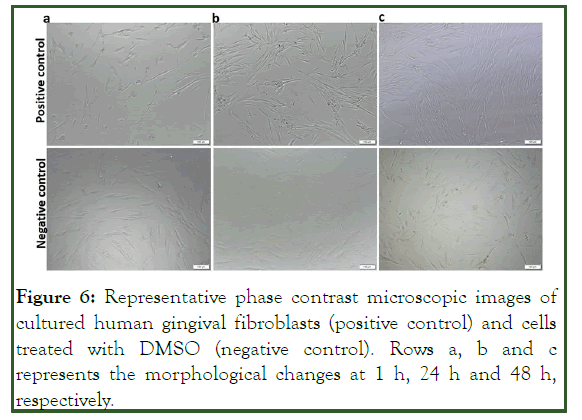
Figure 6: Representative phase contrast microscopic images of cultured human gingival fibroblasts (positive control) and cells treated with DMSO (negative control). Rows a, b and c represents the morphological changes at 1 h, 24 h and 48 h, respectively.
Discussion
Dentin, which is composed of dentinal tubules, is frequently sensitive to external stimuli because of its link in structure and function with the dental pulp. Dentin hypersensitivity develops when the dentinal tubules are exposed to the oral environment and then are subsequently exposed to thermal, tactile, evaporative, chemical, or osmotic stimuli. The wide range of desensitizing agents available and treatment procedures for dentin hypersensitivity can be attributed to the difficulty in managing this condition or to the lack of agreement on the best practices for these treatments. For the treatment of dentin hypersensitivity, there are several products with different components and mechanisms available on the dental market. The purpose of this study was to evaluate the dentinal tubule occluding ability and safety of two different dentin desensitizers: Gluma desensitizer liquid and predicta bioactive desensitizer.
All the specimens in this study were examined using SEM at a magnification of about 5,000X, and photomicrographs were evaluated in order to assess the opening of dentinal tubules in the controls and occlusion of dentinal tubules in specimens treated with the two desensitizing agents. In order to minimize bias, three blind examiners evaluated the SEM images. Score was given to the tubule occlusion on a categorical scale of 0-3, type 0 (no occlusion; score 0), type 1 (partial occlusion; ≤ 25%; score 1), type 2 (partial occlusion; 25-75%; score 2), type 3 (complete occlusion; >75%; score 3). The three reviewers' mean tubule occlusion scores were calculated, and statistical analysis was done using that data as shown in Table 1.
Gluma desensitizer liquid is a composite of 5% Glutaraldehyde and (35%) 2-Hydroxyethyl-Methacrylate (HEMA) and purified water. Schupbach, et al. note that the gluma desensitizer can occlude the dentinal tubules up to 50 μm deep. Furthermore, an earlier study by Mehta et al indicates that gluma desensitizer provides for tubular occlusion by precipitating proteins in the dentin fluid. Results of the current study extends and confirms these former observations (Table 1). Studies conducted using gluma desensitizer has generally stated successful results.
Samuel, et al. in his clinical trial study measured the hypersensitivity of gluma using the schiff sensitivity scale at baseline, immediately, 15 days and 30 days after application and found that the mean hypersensitivity scores were increase towards the end of 30 days. The decreased effectiveness of gluma desensitization may be related to the agent get washed away in saliva, dietary patterns, inadequate acid resistance, and perhaps tooth brushing habits.
In comparison to gluma desensitizer liquid, the predicta bioactive desensitizer which is composed of calcium, phosphate and nanohydroxyapatite, seems to be associated with an efficacious occlusion profile. Luong, et al. also reported that after the application of predicta bioactive desensitizer, SEM micrographs showed good coverage of the widely open dentinal tubule. He also reported that the dentin regions exposed for the experiment were rich in open dentin tubules, and tubular sealing was accomplished by predicta bioactive desensitizer right away, according to the SEM micrographs. Results of this study indicate that the occlusive efficacy of predicta bioactive desensitizer may be similar to that gluma desensitizer liquid (Table 1).
The presence of glutaraldehyde and 2-Hydroxyethyl Methacrylate (HEMA) in gluma desensitizer liquid raises the concerns of cytotoxicity. Ashwini, et al., reported the dentinal tubules can be efficiently blocked by gluma. Although it contains glutaraldehyde, prolonged use can cause gingival damage. As per a study by Eyuboglu, et al. gluma is associated with mean mortality percentages of 96, 94 and 94% for cytotoxicity at 50%, 20% and 10% dilutions, respectively. Ishihata, et al. highlighted the importance of limiting the contact of the desensitizing agent to the target area and preventing inadvertent spreading to neighboring gingival tissue as adverse gingival effects such as inflammation and ulceration may ensure upon inadvertently prolonging the contact of gluma with gingival tissue. Thus, it appears that the gingival safety of gluma desensitizer liquid can be enhanced by meticulous procedural nuances. However, the presence of glutaraldehyde and 2-Hydroxyethyl Methacrylate (HEMA) in gluma desensitizer liquid poses a perceptible “chemical concern”, which may nevertheless be blunted with procedural nuances.
Eyuboglu, et al. also reported that the cytotoxicity of a material is usually related to its composition. Like HEMA, a low molecular weight hydrophilic monomer that may be released from resin-based materials and penetrate the dentin tissue, affecting odontoblast vitality and physiological activity.
Results of our study is in general agreement with the findings of Eyuboglu, et al. indicates that the cytotoxic effects of gluma desensitizer liquid is more pronounced after 24 and 48 hours of application, especially in concentrations higher than 0.1%. In contrast, cell viability with varying concentrations of predicta bioactive desensitizer at 1, 24 and 48 hours remained consistent. The predicta bioactive desensitizer, a composite of highly biocompatible and bioactive materials releases calcium and phosphate ions at the site of treatment to stimulate rapid hydroxyapatite crystal formation within micro cracks in enamel as well as on the surface of treated dentin and in dentinal tubules. Thus, it appears that biocompatibility and gingival safety of the predicta bioactive desensitizer may be better and free from the “chemical concern” discussed above.
Patients with dentin hypersensitivity have a compromised quality of life with respect to dietary choices, dental hygiene, and aesthetics. An optimal treatment modality for dentin hypersensitivity must effectively provide for occlusion of dentinal tubules to reduce fluid movement in dentin and/or reduce the activity of dental sensory nerves along with being acceptably biocompatible and safe with respect with cytotoxicity. Results of the current study indicates that the predicta bioactive desensitizer fares well on this composite requirement and seems to be a good addition to the overall armamentarium against the complications of dentin hypersensitivity. The current study is an in vitro study and additional data from clinical studies may be needed to confirm and extend the findings presented herein.
Conclusion
In the backdrop of a clinical scenario, where there is an apparent lack of a gold-standard, treatment choices have to be arrived on the premise of risk-benefit assessments. While the occlusive benefits of predicta bioactive desensitizer seem comparable to that of gluma desensitizer liquid, the safety concerns with respect to gingival safety may be more favorable with the former. Confirmation of the risk-benefit profiles of the two dentin desensitizers presented herein lends premise for future clinical studies to ascertain the place of predicta bioactive desensitizer as a safe and effective occlusive strategy for managing dentin hypersensitivity.
Conflicts of Interest
The authors of this manuscript certify that they have no proprietary, financial, or other personal interest of any nature or kind in any product, service, and/or company that is presented in this article.
Financial Support/Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
References
- Davari A, Ataei E, Assarzadeh H. Dentin hypersensitivity: Etiology, diagnosis and treatment; a literature review. J Dent (Shiraz). 2013;14(3):136-145.
[Crossref] [Google Scholar] [PubMed]
- Que K, Guo B, Jia Z, Chen Z, Yang J, Gao P. A cross-sectional study: Non-carious cervical lesions, cervical dentine hypersensitivity and related risk factors. J Oral Rehabil. 2013;40(1):24-32.
[Crossref] [Google Scholar] [PubMed]
- Zeola LF, Soares PV, Cunha-Cruz J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J Dent. 2019;81:1-6.
[Crossref] [Google Scholar] [PubMed]
- Bekes K, Hirsch C. What is known about the influence of dentine hypersensitivity on oral health-related quality of life?. Clin Oral Investig. 2013;17:S45-S51.
[Crossref] [Google Scholar] [PubMed]
- Idon PI, Sotunde OA, Ogundare TO. Beyond the relief of pain: Dentin hypersensitivity and oral health-related quality of life. Front Dent. 2019;16(5):325-334.
[Crossref] [Google Scholar] [PubMed]
- Brannstrom M. The hydrodynamic theory of dentinal pain: Sensation in preparations, caries, and the dentinal crack syndrome. J Endod. 1986;12(10):453-457.
[Crossref] [Google Scholar] [PubMed]
- Vallittu PK, Boccaccini AR, Hupa L, Watts DC. Bioactive dental materials-do they exist and what does bioactivity mean?. Dent Mater. 2018;34(5):693-694.
[Crossref] [Google Scholar] [PubMed]
- Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: Recent trends in management. J Conserv Dent. 2010;13(4):218-24.
[Crossref] [Google Scholar] [PubMed]
- Varoni EM, Zuccheri T, Carletta A, Palazzo B, Cochis A, Colonna M, et al. In vitro efficacy of a novel potassium oxalate hydrogel for dentin hypersensitivity. Eur J Oral Sc. 2017;125(2):151-159.
[Crossref] [Google Scholar] [PubMed]
- Schupbach P, Lutz F, Finger WJ. Closing of dentinal tubules by gluma desensitizer. Eur J Oral Sci. 1997;105:414-421.
[Crossref] [Google Scholar] [PubMed]
- Mehta D, Gowda VS, Santosh A, Finger WJ, Sasaki K. Randomized controlled clinical trial on the efficacy of dentin desensitizing agents. Acta Odontol Scand. 2014;72(8):936-941.
[Crossref] [Google Scholar] [PubMed]
- Ishihata H, Finger WJ, Kanehira M, Shimauchi H, Komatsu M. In-vitro dentin permeability after application of gluma® desensitizer as aqueous solution or aqueous fumed silica dispersion. J Appl Oral Sci. 2011;19(2):147-153.
[Crossref] [Google Scholar] [PubMed]
- Samuel SR, Khatri SG, Acharya S, Patil ST. Evaluation of instant desensitization after a single topical application over 30 days: A randomized trial. Aust Dent J. 2015;60(3):336-342.
[Crossref] [Google Scholar] [PubMed]
- Luong MN, Huang L, Chan DC, Sadr A. In-vitro study on the effect of a new bioactive desensitizer on dentin tubule sealing and bonding. J Funct Biomater. 2020;11(2):38.
[Crossref] [Google Scholar] [PubMed]
- Ashwini S, Swatika K, Kamala DN. Comparative evaluation of desensitizing efficacy of dentifrice containing 5% fluoro calcium phosphosilicate versus 5% calcium sodium phosphosilicate: A randomized controlled clinical trial. Contemp Clin Dent. 2018;9(3):330-336.
[Crossref] [Google Scholar] [PubMed]
- Eyuboglu GB, Yeşilyurt C, Erturk M. Evaluation of cytotoxicity of dentin desensitizing products. Oper Dent. 2015;40(5):503-514.
[Crossref] [Google Scholar] [PubMed]
- Al Asmari D, Khan MK. Evaluate efficacy of desensitizing toothpaste containing zinc-carbonate hydroxyapatite nanocrystals: Non-comparative eight-week clinical study. J Int Soc Prev Community Dent. 2019;9(6):566-570.
[Crossref] [Google Scholar] [PubMed]
- dall'Orologio GD, Lone A, Finger WJ. Clinical evaluation of the role of glutardialdehyde in a one-bottle adhesive. Am J Dent. 2002;15(5):330-334.
[Google Scholar] [PubMed]
- Parmar M, Joshi S, Thakkar M, Joshi C, Rupakar P, Desai H. Comparative evaluation of strontium chloride desensitizer and novel proarginin desensitizer on dentinal tubule occlusion-a scanning electron microscopic study. Nat J Integ Res Med. 2016;7(6):85-92.
- Oh S, Gu Y, Perinpanayagam H, Yoo YJ, Lee Y, Kim RK, et al. Dentinal tubule sealing effects of 532-nm diode-pumped solid-state laser, gallic acid/Fe3+ complex, and three commercial dentin desensitizers. Lasers Med Sci. 2018;33(6):1237-1244.
[Crossref] [Google Scholar] [PubMed]
Citation: Alsenan QK, et al (2024) Comparative Assessment of Two Dentin Desensitizers for Dentin Tubule Occlusion and Cytological Safety. Oral Health Dent Manag. 2024;23(3):1121.
Copyright: © 2024 Mustakim F, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
