Indexed In
- Open J Gate
- Genamics JournalSeek
- ResearchBible
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Conference Proceeding - (2020) Volume 9, Issue 2
Colorimetric Analytical Probe for Determination of Formaldehyde and its Validation using a Single Reagent
Anantharaman Shivakumar*Received: 05-Jun-2020 Published: 28-Jun-2020, DOI: 10.35248/2161-1009.20.9.389
Abstract
A simple and sensitive method for the determination of formaldehyde is proposed involving Terbutaline sulphate with the formation of yellow coloured product with absorption maximum at 460 nm in the presence of concentrated sulphuric acid (strength = 36 N) is proposed. The method lies in a calibration range from 0.038 to 0.76 μg ml-1 and molar absorptivity 2.6 × 104 M-1 cm-1. The method has been validated with a Phloroglucinol method in its determination in Fruits, Vegetable and Water bodies.
Keywords
Formaldehyde; Terbutaline sulphate; Single reagent; Validation
Introduction
Formaldehyde, a colourless foul smelling gas is intensively used in the preparation of building materials and household products. It is photo chemically broken down in the Air, generally within hours. It also quickly dissolves in water easily. When dissolved in water in 40% (v/v), called formalin, is used as a disinfectant in several processes for instance in funeral homes and medical labs. It is also used as a preservative in foods, and in Pharma products namely antiseptics, medicines, cosmetics etc. Naturally minute quantities of formaldehyde are always produced during the metabolic processes in human and other living organisms. It can be generated by autoxidation degradation of polyvinylpyrrolidone and polythyleneglycol. It is considered as one of the most significant industrial hazards, air pollutants, and its toxicity to man and animals that has been reported [1,2]. Detection of volatile organic compounds in solution is of supreme importance as it causes a major threat to the environment. Formaldehyde exposure limit of 0.1 ppm is recommended as an indoor air level for all individuals for odour detection and sensory irritation. It has been suggested by International Agency for Research on Cancer (IARC), The National Toxicology Program (NTP), and US Environmental Protection Agency (EPA) that exposure of formaldehyde is associated with nasopharyngeal cancer and leukemia [3-5].
Fomaldehyde have been quantified by Spectrophotometric methods that include Telomerisation reaction, phloroglucinol, HPLC-UV-Vis Spectrophotometry, Crystal-violet-potassium bromate-phosphoric acid, Chromotropic acid-magnesium, Acetylacetone-ammonium acetate, Phenol reagent-acetylacetone, Brilliant green-sulphite. Chromatography methods such as GCMS, High Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Magnetic nanoparticles-HPLC, liquidliquid extraction-LC, Micellar electrokinetic chromatography-2,4- dinitrophenylhydrazine. Hantzsch reaction involving fluorimetry method that includes Acetoacetanilide-, Cyclohexane-1,3-dione, 5,5-dimethylcyclohexane-1,3-dione. Several analytical techniques for the determination of formaldehyde in water and food samples include colorimetric-solid phase extraction, Enzymatic method, HPLC, Gaseous sensor, Colorimetric sensor strips, Nano sized solid phase-kinetic method, Chemiluminiscence, Silver nanoparticlesurface enhanced Raman scattering and so on [6-10].
Bearing all these, the chromatography have serious disadvantages such as, the instruments should be operated by a trained persons only, less economical, error due to overloading of the samples, parts are expensive and sensitive, more solvent for separation, high pressure may be required in times. Furthermore, the fluorescence instrumentation, assay reagents, excitation sources and filters are not economical. Scattering process is one of the serious concerns, as it could contribute to positive interference. All the samples should be deaerated as it can result in erratic or fluctuating readings. The reaction of pH is also a key feature to carry out the reaction or response as it leads to inaccurate measurements.
The proposed analytical methodology adopted is very simple, sensitive compared to some of the available methods already reported. The method uses only single reagent, terbutaline sulphate in an acid medium (conc. H2SO4), which is not only economical but also easily available. The reaction involves the formation of yellow coloured product with a maximum wavelength of absorption at 460 nm due to the coupling and dehydration between two molecules of Terbutaline sulphate in the presence of Formaldehyde and concentrated Sulphuric acid.
Chemicals and Reagent Preparations
All the chemicals that were used in the assay are of analytical grade. Formaldehyde A.R (37%) and terbutaline sulphate A.R (99%) was purchased from Himedia, Mumbai. All the interfering ion solution was tested by using 1000 ppm of the stock. Double distilled water was used throughout the experiment for the preparation of solution and requisite dilution was prepared as per the need. Formaldehyde stock solution (19 ppm) was prepared by 1:1 dilution with water and concentration was determined by carrying out a titration with standard potassium permanganate. 0.2% Terbutaline sulphate was prepared by dissolving 0.2 g in 100 ml of double distilled water.
Fruit, vegetable, plant and water samples were collected locally. The fruit, vegetable and plant were peeled, dried at 300C for 2 h, weighed and homogenised using a blender and diluted with sufficient quantity of double distilled water and squeezed using a muslin cloth. This solution was used as a stock. The suitable dilution was prepared as per the calibration graph. Aqueous extract was prepared just before the start of the experiments so as to prevent undesired degradation reaction, then assayed at least in triplicate and results are average out. All the infusion were analysed as fresh as possible for reliability of the results. The water samples were collected nearby ponds of Mysore city in Karnataka, India [11-15].
Instrumentation
A SYSTRONICS (2202, India) PC based double beam ultravioletvisible (UV-Vis) spectrophotometer with 1.0 cm matched cells was used for all absorbance measurements. All the glass apparatus were neatly washed with a mixture of potassium dichromate and sulphuric acid during each step of the optimisation followed by tap water and rinsed thoroughly with double distilled water to avoid unwanted side reaction [16-20].
Optimisation of the Reaction Condition and Reagents for the Colour Development
Optimisation of the reaction condition was carried out by onefactor- at-a-time method. An attempt was made to carry out the reaction in various concentrations of sulphuric acid (2 N to concentrated). Finally it was seen that the use of concentrated sulphuric acid showed a maximum colour development (Graphical plot not shown). 0.5 ml of Terbutaline sulphate solution (0.2%) was needed for the development of maximum colour. Higher concentration gave lesser absorption values.
Results and Discussions
Preparation of the calibration curve
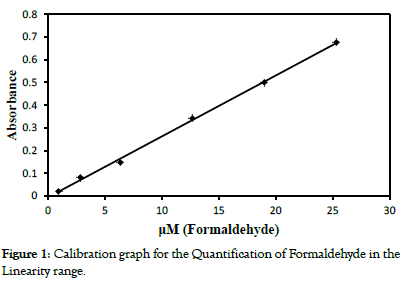
To a series of 10 ml standard flask, aliquots containing 0.38 to 7.6 μg formaldehyde solutions, resulting in the concentration range between 0.038 and 0.76 μg ml-1 (in terms of molar concentrations 0.9 to 25 μM), was added. To each of the standard flask, 0.5 ml of terbutaline sulphate and 4.0 ml concentrated sulphuric acid was added. The mixture was shaken well and diluted to volume by double distilled water. The developed yellow colour, after cooling, instantaneously was measured at 460 nm against the reagent blank containing all the reagents, except formaldehyde. The reproducibility of the calibration graph was checked by carrying out a triplicate analysis. The unknown concentration of formaldehyde was determined by the calibration graph. The Table 1 indicates the spectral characteristics of the coloured product (Figure 1).
Figure 1: Calibration graph for the Quantification of Formaldehyde in the Linearity range.
Table 1: Optical parameters for the Determination of Formaldehyde.
| Parameters | Characteristics |
|---|---|
| Colour | Yellow |
| λmax | 460 nm |
| Stability | 5 hrs |
| Beer’s law range (μg ml-1) | 0.038-0.76 |
| Molar absorptivity (M-1cm-1) | 2.6 x 104 |
| Detection limita | 0.009 |
| Limit of quantification | 0.030 |
| Regression equation (b) | |
| Correlation co-efficient | 0.998 |
| Slope (a) (μM)-1 cm-1 | 0.026 |
| Intercept (b) | -0.005 |
| Relative standard deviationc (%) | 2.0 |
| Reaction time | Instantaneous |
(a) Detection limit=(3.3*SDblank)/slope of calibration.
(b) Y=ax+b where x is concentration in μM and Y is absorbance.
(c) Three replicate measurements.
Effect of temperature and time
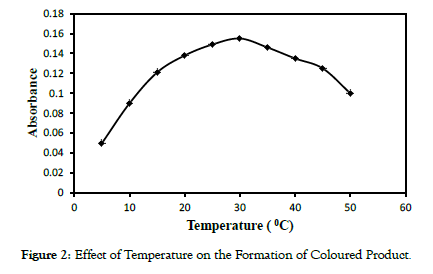
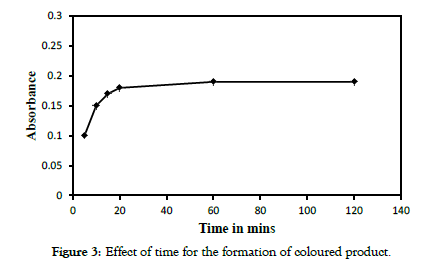
The effect of temperature and time for the formation of coloured product was studied at 6.3 μM formaldehyde concentration. It was observed that the intensity of absorption decreases beyond 30 °C which can be attributed to the volatisation of formaldehyde. Figure 2 (refer figure numbers in chronological order) depicts the effect of temperature under the reaction condition. Effect of time studied found that formation of the coloured product was instantaneous, reaches a maximum at 20 mins beyond which remains stable up to 5 hrs which is sufficiently enough for the quantitative determination (Figure 3).
Figure 2: Effect of Temperature on the Formation of Coloured Product.
Figure 3: Effect of time for the formation of coloured product.
Proposed reaction mechanism
The proposed reaction mechanism is suggested in step wise as described below in the Scheme 1,
Step 1: Formation of protonated form of formaldehyde.
Step 2: Electrophillic substitution of Terbutaline sulphate.
Step 3: Elimination of water to result in stable aromatic product.
Step 4: Process of dimerization.
Step 5: Removal of proton form the product in the step 4 to diphenylmethane derivative.
Step 6: Formation of delocalised tautomer.
Effect of foreign chemicals
Interference from foreign substances was tested by analyzing their effect in a fixed concentration of 0.57 μg ml-1 of formaldehyde. The experimental results are tabulated in the Table 2. It has been seen that the method has a minimum interference from alcohol, acetone, acetaldehyde, dextrose which are part of the analysis in fruit, and vegetables. It also recorded that except nitrate and magnesium, other chemicals used in the reaction did not interfere significantly with the proposed method. Nitrate interference was suitably eliminated by pre-treatment of the solutions with Copperised zinc and treating the solution with 0.5% Sulphamic acid. The minimum interference from carbonyl containing species such as acetone, acetaldehyde, and dextrose clearly indicates the reaction is highly specific for formaldehyde [21-31].
Table 2: Effect of interfering species on the determination of Formaldehyde.
| Foreign species | Tolerance limit* (μg/mL) |
|---|---|
| Alcohol | 25000 |
| Acetone | 20000 |
| Acetaldehyde | 5000 |
| Dextrose | 6000 |
| Sucrose, lactose | 5000 |
| Oxalate, acetate, Ascorbic acid | 4000 |
| Na+, phosphate | 2500 |
| Zn2+, sulphate, sulphite, | 1000 |
| Co2+, tartarate | 900 |
| Fe2+, Ca2+, carbonate | 850 |
| Ni2+, Cl-, F- EDTA, I- | 725 |
| Mn2+, Cu2+ , Hg2+ | 450 |
| Fe3+, dichromate, Ce4+, Al3+ | 300 |
| Nitrate** | 100 |
| Magnesium | 4 |
*Tolerence limit of interfering species was established at the concentration that do not cause error more than ± 3% in absorbance values at 0.57 μg ml-1 formaldehyde concentration.
**Eliminated by treating the solution with Copperised zinc and 0.5% Sulphamic acid.
Application for the determination of formaldehyde in fruit, vegetables and water
The proposed reaction in the determination was applied for formaldehyde quantification in the spiked fruit, vegetables and water samples. The percentage recoveries were reported as presented in the Table 3. The recovery percentage lies in the range from 95.3 to 103.3 and 98.9 to 102.3 for the proposed and reported methods, respectively.
Table 3: Determination of Formaldehyde in Fruit, Vegetables and Water.
| No | Samples tested | Present method %Recovery ±R.S.D |
Reported method % Recovery ±R.S.D [7] |
|---|---|---|---|
| A | Fruits | ||
| 01 | Malus domestica | 95.3 ± 0.9 | 99.6 ± 0.45 |
| 02 | Citrus limon | 97.3 ± 1.4 | 101.1 ± 0.56 |
| 03 | Musa paradisiaca | 103.3 ± 2.5 | 100.5 ± 0.36 |
| 04 | Averrhoa carambola | 96.8 ±0.90 | 102.3 ± 0.29 |
| 05 | Manilkara zapota | 101.8 ± 0.96 | 99.6 ± 0.46 |
| 06 | Citrullus lanatus | 99.9 ± 0.89 | 99.1 ± 0.56 |
| B | Vegetables | ||
| 01 | Daucus carota | 98.7 ± 1.9 | 98.9 ± 0.29 |
| 02 | Cucurbita pepo | 97.6 ± 1.56 | 101.3 ± 0.89 |
| 03 | Moringa oleifera | 96.8 ± 1.89 | 102.3 ± 0.92 |
| C | Water sample from the local water bodies | 95.6 ± 0.78 | 101.8 ± 0.86 |
Determination has been made after adding three different amounts of formaldehyde in triplicates to the fixed concentration of pre-analysed analyte.
Conclusion
A colorimetric method has been proposed for the determination of formaldehyde by using terbutaline sulphate has been reported. The method involves a single reagent, which involves the intra molecular coupling of terbutaline sulphate in the presence of concentrated sulphuric acid. The linearity of the proposed assay lies in the range of 0.038 to 0.76 μg ml-1. The method has been successfully applied for formaldehyde determination in fruits, vegetables and local water bodies samples with recovery ranging between 95.3 to 101.8%. As formaldehyde, a serious pollutant in the water bodies, household edible products and its colorimetric quantification makes a great impact on the scientific community. The proposed assay would thus be a method which can be adopted in economically weaker analytical laboratories.
Acknowledgements
Author Dr. Anantharaman Shivakumar (A.S.K) thanks St. Philomena’s College for providing facilities to carry out research.
REFERENCES
- Gullapalli RP, Mazzitelli CL. Polyethylene glycols in oral and parenteral formulations—A critical review. Inl J Pharmaceutics. 2015;496:219-239.
- Pandey S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J Science: Advanced Materials and Devices. 2016;1:431-453.
- Pandey S, Nanda KK. Au Nanocomposite Based Chemiresistive Ammonia Sensor for Health Monitoring. ACS Sensors. 2016;1:55-62.
- Pandey S, Goswami GK, Nanda KK. Nanocomposite based flexible ultrasensitive resistive gas sensor for chemical reactions studies. Scientific Reports. 2013;3: 2082.
- Golden R. Identifying an indoor air exposure limit for formaldehyde considering both irritation and cancer hazards. Critical Reviews in Toxicology. 2011;41:672-721.
- Yasri NG, Seddik H, Mosallb MA. Spectrophotometric determination of formaldehyde based on the telomerization reaction of tryptamine. Arabian J Chemistry. 2015;8:487-494.
- Gayathri N, Balasubramanian N. Spectrophotometric Determination of Formaldehyde. Analytical Letters. 2000;33:3037-3050.
- Sun HH, Mo XM, Qian YM. Comparison of HPLC and Spectrophotometry Methods on Quantitative Determination of Formaldehyde in Water Based Coatings. Advanced Materials Res. 2013;816-817.
- Yue XF, Zhang ZQ. Simultaneous determination of formaldehyde and methanol by flow-injection catalytic spectrophotometry. J Analytical Chemistry.2007;62:992-996.
- Gasparini F, Weinert PL, Lima LS, Pezza L, Pezza HR. A simple and green analytical method for the determination of formaldehyde. J Brazilian Chemical Society. 2008;19:1531-1537.
- Nassiri M, Kaykhaii M, Hashemi SH, Sepahi M. Spectrophotometric Determination of Formaldehyde in Seawater Samples after In-situ Derivatization and Dispersive Liquid-Liquid Microextraction. Iranian J Chemistry and Chemical Engineering (IJCCE). 2018;37:89-97.
- Jin S, Wang H, Li H, Liu H. The Comparison between Chemical Spectrophotometry and Instrument Method to Determine the Formaldehyde Concentration of the Indoor Air. In 2012 Int Conference on Biomedical Engineering and Biotechnology. 2012;1787-1790.
- Bolognesi L, Santos EJ, Abate G. Determination of formaldehyde by flow injection analysis with spectrophotometric detection exploiting brilliant green–sulphite reaction. In Chemical Papers. 2015;791.
- Yeh TS, Lin TC, Chen CC, Wen HM. Analysis of free and bound formaldehyde in squid and squid products by gas chromatography–mass spectrometry. J Food and Drug Analysis. 2013;21:190-197.
- Bagheri H, Ghambarian M, Salemi A, Es-Haghi A. Trace determination of free formaldehyde in DTP and DT vaccines and diphtheria–tetanus antigen by single drop microextraction and gas chromatography–mass spectrometry. J Pharmaceutical and Biomedical Analysis. 2009;50:287-292.
- Qbal MZ, Novalin S. Analysis of formose sugar and formaldehyde by high-performance liquid chromatography. J Chromatography A. 2009;1216:5116-5121.
- Hu HC, Tian YX, Chai XS, Si WF, Chen G. Rapid determination of residual formaldehyde in formaldehyde related polymer latexes by headspace gas chromatography. J Industrial and Engineering Chemistry. 2013;19:748-751.
- Safari M, Yamini Y, Tahmasebi E, Latifeh F. Extraction and preconcentration of formaldehyde in water by polypyrrole-coated magnetic nanoparticles and determination by high-performance liquid chromatography. J Separation Science. 2015;38:3421-3427.
- Miralles P, Chisvert A, Alonso MJ, Hernandorena S, Salvador A. Determination of free formaldehyde in cosmetics containing formaldehyde-releasing preservatives by reversed-phase dispersive liquid–liquid microextraction and liquid chromatography with post-column derivatization. J Chromatography A. 2018;1543:34-39
- Xu LN, Gai FY, Mu GF, Gao Y, Liu HT, et al. Determination of formaldehyde in aquatic products by micellar electrokinetic capillary chromatography with 2,4-dinitrophenylhydrazine derivatization. Acta Chromatographica. 2012;24:519-528.
- Li Q, Sritharathikhun P, Motomizu S. Development of Novel Reagent for Hantzsch Reaction for the Determination of Formaldehyde by Spectrophotometry and Fluorometry. Analytical Sciences. 2007;23:413-417.
- Zhao XQ, Zhang ZQ. Microwave-assisted on-line derivatization for sensitive flow injection fluorometric determination of formaldehyde in some foods. Talanta. 2009;80:242-245.
- Sakai T, Tanaka SI, Teshima N, Yasuda S, Uran N. Fluorimetric flow injection analysis of trace amount of formaldehyde in environmental atmosphere with 5,5-dimethylcyclohexane-1,3-dione. Talanta. 2002;58:1271-1278.
- Hill AA, Lipert RJ, Fritz JS, Porter MD. A rapid, simple method for determining formaldehyde in drinking water using colorimetric-solid phase extraction. Talanta. 2009;77:1405-1408.
- Sibirny V, Demkiv O, Klepach H, Honchar T, Gonchar M. Alcohol oxidase- and formaldehyde dehydrogenase-based enzymatic methods for formaldehyde assay in fish food products. Food Chemistry. 2011;127:774-779.
- Zhao J, Wang G, Cao T, Guo Z. Development of a Novel Derivate Assay for Formaldehyde Determination by HPLC in Beer Samples. Food Analytical Methods. 2016;9:156-163.
- Monkawa A, Gessei T, Takimoto Y, Jo N, Wada T, Sanari N. Highly sensitive and rapid gas biosensor for formaldehyde based on an enzymatic cycling system. Sensors and Actuators B: Chemical. 2015;210:241-247.
- Wang X, Si Y, Mao X, Li Y, Yu J, Wang H, et al. Colorimetric sensor strips for formaldehyde assay utilizing fluoral-p decorated polyacrylonitrile nanofibrous membranes. Analyst. 2013;138:5129-5136.
- Afkhami A, Bagheri H. Preconcentration of trace amounts of formaldehyde from water, biological and food samples using an efficient nanosized solid phase, and its determination by a novel kinetic method. Microchim Acta. 2012;176:217-227.
- Akshath US, Sagaya Selvakumar L, Thakur MS. Detection of formaldehyde in food samples by enhanced chemiluminescence. Analytical Methods. 2012;4:699-704.
- Ma P, Liang F, Wang D, Yang Q, Ding Y, Yu, et al. Ultrasensitive determination of formaldehyde in environmental waters and food samples after derivatization and using silver nanoparticle assisted SERS. Microchim Acta. 2015;182:863-869.
Citation: Shivkumar A (2020) Colorimetric Analytical Probe for Determination of Formaldehyde and its Validation using a Single Reagent. Biochem Anal Biochem; 9:389. doi: 10.35248/2161-1009.19.8.389
Copyright: © 2020 Shivkumar A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.