Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 8, Issue 4
Chest CT versus RT-PCR for Diagnostic Accuracy of COVID-19 Detection: A Meta-Analysis
Daisy Young1, Liana Tatarian1, Ghulam Mujtaba2, Priscilla Chow3, Samer Ibrahim1, Gunjan Joshi1, HaarisNaji4, Phillip Berges5, Krishna Akella6, Howard Sklarek1, Kashif Hussain1 and Akella Chendrasekhar6*2Milwaukee, WI, USA
3Norristown State Hospital Department of Internal Medicine, Norristown, PA, USA
4Arrowhead Regional Medical Center, Colton, CA, USA
5St. Charles Hospital, Port Jefferson, NY, USA
6Richmond University Medical Center, Staten Island, NY, USA
Received: 08-May-2020 Published: 15-Jun-2020
Abstract
Background: The rapid outbreak of COVID-19 has necessitated expedient methods of detection to prevent further spread and mortality from the virus. Currently, RT-PCR is considered the gold standard. However, its diagnostic priority compared to Chest CT remains unknown. Objective: We sought to perform a meta-analysis using retrospective studies comparing Chest CT and RT-PCR in COVID-19 detection among hospitalized patients. Methods: We performed a comprehensive literature search using Pubmed and Google Scholar for studies comparing Chest CT and RT-PCR between January 1 and April 3, 2020. Outcomes included COVID-19 detection using RT-PCR alone, Chest CT alone, true positives when combining the two, and true negatives when combining the two. Results were reported as an odds ratio (OR) with 95% CI. Results: A total of 6 retrospective studies were included comparing RT-PCR with Chest CT. A total of 1,400 patients were enrolled (average age 46.28 ± 2.7 years, 41.6% were males). Chest CT was superior to RT-PCR for COVID-19 detection [OR 3.86, 95% CI (1.79- 8.31, p=0.0006)]. Heterogeneity (I2) was high (75%), but sensitivity analysis failed to reveal any single contributor to observed heterogeneity. Conclusion: Chest CT appears to be a more sensitive and quicker alternative to RT-PCR in the detection of COVID-19 in hospitalized patients, and may serve as a superior screening tool.
Keywords
Coronavirus; COVID-19; SARS-CoV2; Computed tomography; Reverse transcription polymerase chain reaction
Introduction
Corona viruses are a family of enveloped, single-stranded RNA viruses, of which seven known human corona viruses (HCoVs) have been identified.1 Four commonly detected strains include 229E, OC43, NL63 and HKU1, which have relatively low virulence in humans [1,2]. The three other strains of HCoVs confer higher mortality rates in human populations due to its unique pathogenicity, overwhelming systemic response, and lack of effective treatments. These include severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Mortality rates vary among these three strains: MERS-CoV is 34%, SARS-CoV1 is 11% and SARS-CoV-2 (COVID-19) is currently 3.4% [3,4]. As of April 4, 2020, there are over 1.05 million confirmed cases globally with 59,985 deaths [5].
Person-to-person spread of COVID-19 is thought to be primarily transmitted via respiratory droplets with a median incubation period of four days [6]. Currently, reverse-transcription polymerase chain reaction (RT-PCR) is considered the gold standard for COVID-19 detection. RT-PCR tests usually take just a few hours to complete, but are limited by the time required to transport and prepare samples for testing. As RT-PCR testing has been exponentially increasing, labs have been inundated with samples resulting in significantly delayed diagnostic times and increased usage of personal protective equipment (PPE) amidst an international shortage. The total positive rate of RT-PCR was reported to be about 30% to 90% at initial presentation depending on the respiratory site [7]. Given the current global emergency, the variable sensitivity of RT-PCR and long wait times for results imply that many patients afflicted with COVID-19 may not be identified, risking further infection to healthy populations. Chest computed tomography (Chest CT) is a quick test to perform and may aid in the diagnosis of COVID-19, especially in the current climate of overrun laboratories.
Hence, we performed a Meta analysis to evaluate the clinical utility and sensitivity of Chest CT in comparison to RT-PCR.
Methods
Search strategy
We searched Pubmed and Google Scholar regarding abstracts and manuscripts using key words: COVID-19 detection OR COVID-19 AND “Chest CT,” and COVID-19 AND “RT-PCR,” AND COVID-19 AND “PCR” from January 1, 2020 to April 3, 2020.
Study selection
The eligibility criteria for included studies were as follows:
1. All retrospective studies reporting clinical outcomes comparing Chest CT and RT-PCR.
2. Human subjects of all ages.
3. Writing in English language.
Wrong technology, duplicates, population size under 10, retracted manuscripts, manuscripts without PCR data, editorials, and systematic reviews were excluded (Figure 1).
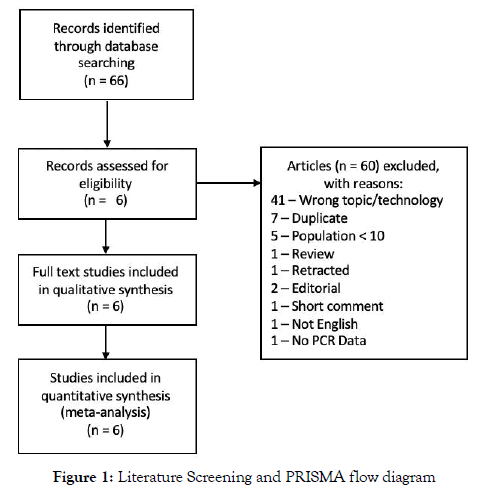
Figure 1: Literature Screening and PRISMA flow diagram
Data extractions
Two investigators (DY and LT) independently performed the literature search and screened all titles and full text versions of all relevant studies that met study inclusion criteria. Any discrepancies between the two investigators were resolved with consultation with the senior investigator (AC). Data obtained included: Title, year of publication, type of study, sample size, average age, sex, and timing of RT-PCR in relation to hospitalization. Quantitative data of COVID-19 detection including discrete numbers of detection between RT-PCR and Chest CT were obtained (Figure 2). A funnel plot was used to assess publication bias (Figure 3).
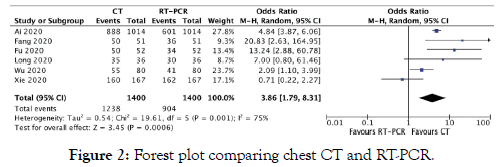
Figure 2: Forest plot comparing chest CT and RT-PCR.
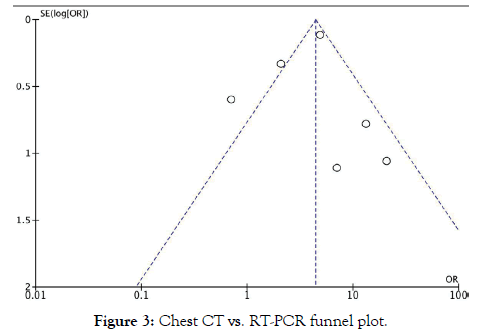
Figure 3: Chest CT vs. RT-PCR funnel plot.
Outcomes
The primary outcome evaluated in our study was accuracy of COVID-19 detection. Accuracy was determined with concomitant Chest CT and RT-PCR usage.
Statistical analysis
Statistical analysis for OR estimate of each study was calculated using Cochrane RevMan version 5.3 (Cochrane Collaboration, London, United Kingdom). Results were expressed as an odds ratio (OR) with a 95% confidence interval (CI). The OR estimate of each study was calculated by the random-effects model obtained by the DerSimonian method [8]. Higgins I-squared (I2) was used to quantify heterogeneity (I2 <50% was defined as low) [9]. P <0.05 was considered statistically significant. Sensitivity analysis was performed to assess significant heterogeneity (I2 >50%) discerning individual contribution to the aggregate. Funnel plots were also used in conjunction with sensitivity analysis to better assess publication bias.
Results
Search results
A total of 66 potentially relevant citations were identified from initial screening. After a detailed evaluation of these studies, 6 studies ultimately met the criteria enrolling a total of 1,400 patients (Figure 1).
Study characteristics
This meta-analysis evaluates COVID-19 detection efficacy in RTPCR in comparison to Chest CT. A total of 6 retrospective studies were included totaling 1,400 patients enrolled. All of the included studies are from China. The average age of the patients included in the studies was 46.28 ± 2.7 years. All studies used RT-PCR on initial presentation to the hospital and included some patients that had serial RT-PCR tests. RT-PCR was the standard for comparison. The location of respiratory site included the throat, sputum, nasopharyngeal, and mouth. The Newcastle-Ottawa Quality Assessment Scale (NOS) was used for qualitative evaluation of the included studies (Tables 1 and 2).
Table 1: Qualitative evaluation of included studies using Newcastle-Ottawa scale.
| Study | Selection (max 4 stars) | Comparability (max 2 stars) | Outcome (max 3 stars) |
|---|---|---|---|
| Ai | *** | * | *** |
| Fang | *** | * | ** |
| Fu | *** | * | *** |
| Long | *** | * | ** |
| Wu | *** | * | ** |
| Xie | *** | * | ** |
Table 2: Study characteristics.
| Study | Ai | Fang | Fu | Long | Wu | Xie |
|---|---|---|---|---|---|---|
| Year | 2020 | 2020 | 2020 | 2020 | 2020 | 2020 |
| Type | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Average Age (in years) | 51 | 45 | 44.5 | 44.8 | 46.1 | Not recorded |
| # Enrolled | 1014 | 51 | 52 | 36 | 80 | 167 |
| % Males | 46.1% | 59.6% | 53.8% | 55.6% | 48.8% | Not recorded |
| Timing of PCR | Initial, some serial | Initial, some serial | Initial, some serial | Initial, some serial | Initial, some serial | Initial, some serial |
| PCR Location | Throat | 45throat, 6sputum |
Nasopharyngeal | Oral, Nasal | Throat, Nasal | Oral |
| Standard for Comparison | PCR | PCR | PCR | PCR | PCR | PCR |
Primary outcome
COVID-19 Detection: A total of 6 retrospective studies were included comparing RT-PCR and Chest CT [10-15]. A total of 1,400 patients were enrolled (average age 46.28 ± 2.7 years, 41.6% males). Overall, Chest CT was superior to RT-PCR for COVID-19 detection [OR 3.86, 95% CI (1.79-8.31, P=0.0006)].
All studies except for Xie et al favored CT chest over RT-PCR. Xie et al found that Chest CT was positive in 160 patients while RT-PCR was positive in 162 patients of a total sample of 167 patients.15 Ai et al was the only study that found true negatives that were consistent between studies [10].
Publication Bias: In addition to sensitivity analysis reported below, publication bias was assessed using a funnel plot (Figure 3) revealing an asymmetrical distribution. This confirms observed heterogeneity and explains the absence of change in heterogeneity noted on sensitivity analysis. Findings should be considered critically and within the context of resource limitation.
Sensitivity analysis: Due to the significant heterogeneity observed in primary outcome, sensitivity analysis was performed by excluding one study at a time to see if any had a significant contribution to observed heterogeneity. The heterogeneity found may be associated with difference in viral load or disease severity, difference in evaluation among the included studies, other institutional variations and is discussed further in the limitations section. A modified plot for sensitivity analysis was not included since the exclusion of any single study demonstrated no significant changes observed in heterogeneity (I2=75%).
Discussion
Main findings
Our analysis found that an initial CT chest was more effective than RT-PCR in the detection of COVID-19 among hospitalized patients.
Clinical implications
The rapid outbreak of the novel coronavirus (COVID-19) which has since become a pandemic has necessitated expedient methods of detection to prevent further spread and mortality from the virus. In the appropriate clinical setting where the index of suspicion for COVID-19 is high, namely patients with new onset fever and/or respiratory tract symptoms, travel within the prior 14 days to a location where there is COVID-19, or close contact with a confirmed or suspected case of COVID-19 in the prior 14 days, in conjunction with common laboratory findings (such as lymphopenia, elevated inflammatory markers, elevated liver enzymes, etc.), the use of a quick and accurate screening tool is paramount [15,16]. While RT-PCR is considered the gold standard for definitive diagnosis, there are limitations to its availability, sensitivity for detection COVID-19, and extended waiting times for results. Furthermore, inter-operator variability could also affect the quality of sample obtained and result in a false negative. To counteract this limitation and monitor recovery from COVID-19, serial RT-PCR measurements could potentially be beneficial; however, current resource limitations prevent repetitive testing from occurring.
Factoring in the financial burden of increased hospital length of stay and use of PPE, Chest CT is a rapid and cost-effective alternative to RT-PCR. Among COVID-19 patients, Chest CT demonstrates typical radiographic features including ground-glass opacities, multifocal patchy consolidation, and/or interstitial changes with a peripheral distribution [17]. These findings, in conjunction with a high clinical suspicion, confer a highly specific diagnosis for COVID-19.
Based on prior clinical reports, we theorize that as our understanding of the clinical progression of the SARS-CoV2 viral load becomes more comprehensive, we may find that severity of symptoms and immune response has an inverse relationship to viral load, similar to HIV infection. Given our study findings in the setting of resource limitations of a surge, we believe that the primary role for RT-PCR would be in:
» Community level detection prior to hospitalization.
» Confirmatory test for Chest CT, particularly in setting of potential co-infection.
» A tool for subsequent evaluation of patients with absence of chest CT findings with a high clinical index of suspicion.
Until further clarification can be provided through the constantly evolving clinical picture, the ideal time period for viral load measurement and re-measurement have not been uniformly agreed upon.
Study Limitations: There are several limitations to the performed meta-analysis to consider.
1. Variance in viral load related to disease severity
2. Variance in viral load depending on location obtained
3. Different test kits
4. Instability of RNA
5. Difficulty of viral RNA extraction
6. Variability in interval of serial testing
7. Location of testing
8. Different risk factors
9. Different severity of illness
10. Variability of primers used
11. Onset of symptoms prior to hospitalization
12. Variance in age
13. Variance in other population severity risk factors
14. Study by Ai et al accounts for 1014 of 1400 patients
15. Study by Ai et al was the only study that had true negatives for both Chest CT and RT-PCR.
16. All studies were taken from Chinese studies; hence further studies in other countries are needed to corroborate these findings.
Further evaluation with a large-scale, head-to-head trial directly comparing RT-PCR and Chest CT with better uniformity of testing intervals, testing locations, and similar points in disease progression would further elucidate the efficacy and shortcomings of either modality. In the absence of such a trial, our study helps clarify collective differences observed. Additional testing performed in other countries would better inform clinicians as to variability based on region.
Conclusion
RT-PCR is currently regarded as the gold standard for COVID-19 detection. The results of our Meta analysis indicate that Chest CT appears to be a more sensitive and quicker alternative to RT-PCR in the detection of COVID-19 in hospitalized patients. Chest CT may serve as a superior screening tool to RT-PCR, particularly in the setting of resource limitation.
REFERENCES
- Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490-502.
- Isaacs D, Flowers D, Clarke JR, Valman HB, MacNaughton MR. Epidemiology of coronavirus respiratory infections. Arch dis Child. 1983;58(7):500-503.
- Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020.
- Rajgor DD, Har Lee M, Archuleta S, Bagdasarian N, Chye Quek S, The many estimates of the COVID-19 case fatality rate. The Lancet Infectious Diseases. 2020.
- Coronavirus disease 2019 (COVID-19) Situation Report -75. World Health Organization. 2020.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199-1207.
- Yang Y, Yang M, Shen C, Wang F, Yuan J, Li J, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. 2020;11:20021493.
- DerSimonian R, Laird N, Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
- Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of CHest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;26:200642.
- Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;19:200432.
- Fu H, Xu H, Zhang N, Xu H, Li Z, Chen H, et al. Association between Clinical, Laboratory and CT Characteristics and RT-PCR Results in the Follow-up of COVID-19 patients. medRxiv. 2020;19:20038315.
- Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;25:108961.
- Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical Characteristics of Imported Cases of COVID-19 in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020;29:ciaa199.
- Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J, et al. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020 Feb 12:200343.
- Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295(1):202-207.
- Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. 2020.
Citation: Young D, Tatarian L, Mujtaba G, Chow P, Ibrahim S, Joshi G, et al. (2020) Chest CT versus RT-PCR for Diagnostic Accuracy of COVID-19 Detection: A Meta-Analysis. J Vasc Med Surg 8:3. doi: 10.35248/2329-6925.20.8.392.
Copyright: © 2020 Chendrasekhar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

