Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2024) Volume 15, Issue 4
Cardiovascular Events Post COVID-19 Vaccination: A Systematic Review and Bayesian Multivariate Meta-Analysis of Observational Studies
Raheleh Karimi1,2, Mina Norozirad3, Foad Esmaeili4 and Marjan Mansourian2,5*2Department of Biostatistics and Epidemiology, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran
3Department of Statistics, Center for Mathematics and Applications (NOVA Math), NOVA School of Science and Technology (NOVA SST), 2825-149 Caparica, Portugal
4Department of Statistics, Mathematics and Computer, Allameh Tabataba’i University, Tehran, Iran
5CREB, Universitat Politècnica de Catalunya, BarcelonaTech (UPC), Building H, Floor 4, Av. Diagonal 647, 08028 Barcelona, Spain
Received: 29-May-2024, Manuscript No. JVV-24-25882 ; Editor assigned: 31-May-2024, Pre QC No. JVV-24-25882(PQ); Reviewed: 19-Jun-2024, QC No. JVV-24-25882 ; Revised: 26-Jun-2024, Manuscript No. JVV-24-25882 (R); Published: 03-Jul-2024, DOI: 10.35248/2157-7560.24.15.559
Abstract
Background: With the aim of providing a detailed understanding and applying a comprehensive strategy, this study examines the association between COVID-19 vaccination and cardiovascular events.
Methods: We conducted a Bayesian multivariate meta-analysis using summary data across multiple outcomes including myocardial infarction, stroke, arrhythmia and CAD, considering potential dependencies in the data. Markov chain Monte Carlo (MCMC) methods was detected for easy implementation of the Bayesian approach. Also, the sensitivity analysis of the model was done by using different priors.
Results: Fifteen studies were included in the systematic review, with eleven studies comparing the results between the vaccine group and the unvaccinated group. Additionally, six studies were used for further analysis to compare mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna). Bayesian meta-analysis revealed a link between vaccines and CAD risk (OR, 1.70; 95% CrI: 1.11–2.57), particularly after BNT162b2 (OR, 1.64; 95% CrI: 1.06-2.55) and second dose (OR, 3.44; 95% CrI: 1.99-5.98). No increased risk of myocardial infarction, arrhythmia, or stroke post-COVID-19 vaccination was found. Secondary analysis showed no notable disparity in cardiovascular outcomes between BNT162b2 and mRNA vaccines.
Conclusions: The association of COVID-19 vaccination with the risk of coronary artery disease should be considered in future vaccine technologies for next pandemics.
Keywords
COVID-19 vaccines; Stroke; Myocardial infarction; Arrhythmias; Cardiac; Coronary artery disease; SARSCoV-2
Introduction
As of 8 November, 2023, the World Health Organization reported that there have been over 771820937 confirmed cases of COVID-19 worldwide, resulting in 6978175 deaths [1]. Vaccines have played an important role in controlling and preventing the spread of COVID-19 by helping develop immunity in individuals, thus lowering the risk of severe illness and infection [2]. To date, more than 11.8 billion vaccine doses have been distributed globally [3].
However, despite the success of vaccination campaigns, several issues have been linked to the COVID-19 vaccines, particularly worries regarding cardiovascular complications, which have garnered attention [4-7]. It is essential to tackle these allegations and provide clarity on the true effects of the vaccines on heart health, as well as ease individuals’ anxieties related to such worries. Concerns regarding the potential health risks linked to vaccines may overshadow a logical evaluation of the advantages of vaccination and result in skepticism towards vaccines in upcoming pandemics. Hence, it is important to address these claims and offer scientific clarifications to alleviate worries and regain public trust in COVID-19 vaccines.
The findings of several studies in this field have reported conflicting results about the effect of COVID-19 vaccines on cardiovascular events. Some findings show that the use of these vaccines may increase the incidence of stroke, myocardial infarction and arrhythmia [4,8,9]. On the other hand, specific research has shown that vaccines can have significant benefits in preventing some cardiovascular events such as myocardial infarction and stroke [10-12]. Also, some studies have shown that there is no significant association between COVID-19 vaccines and cardiovascular events [13,14]. Therefore, a comprehensive review or meta-analysis is needed to draw reliable conclusions regarding the effects of the corona vaccine on cardiovascular health.
Several systematic reviews and meta-analyzes have assessed the cardiovascular event risk following COVID-19 vaccination. However, the focus has mainly been on issues like myocarditis and pericarditis [15-18]. Uncertainty remains regarding other complications such as arrhythmia, stroke, Coronary Artery Disease (CAD) and Myocardial Infarction (MI) [19,20]. Furthermore, many of these studies are based on case reports and case series without control group comparisons. It is difficult to assess the link between vaccination and cardiovascular events solely through case reports and population-based data could offer more accurate estimates. Additionally, no research has explored the connection between CAD events. A thorough study is required to analyze various cardiovascular outcomes concurrently and contrast the findings with those of a control group.
In this study, our goal is to present a strong Bayesian multivariate meta-analysis model to examine the link between vaccine-related cardiovascular events in controlled studies, taking into account correlations between outcomes. This method enables people to make informed decisions about their health and enhances public confidence in vaccination programs, thereby supporting public health and the management of infectious diseases.
Materials and Methods
Objectives
The primary goal is to examine the possible presence of cardiovascular events, specifically myocardial infarction, CAD, arrhythmia and stroke, linked to COVID-19 vaccination. Additionally, the aim is to provide comprehensive details on the demographic and clinical characteristics of both vaccinated and unvaccinated groups, in order to perform subgroup analysis to more effectively explore the main objective. In this study, we compared BNT162b2 and mRNA vaccines, with a focus on cardiovascular complications as a Secondary Analysis.
Protocol
The review adheres to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) guidelines for systematically reviewing the existing literature [21].
Search strategy
A thorough search of prominent electronic databases (such as PubMed, Web of Science, Scopus, Cochrane Library and Google Scholar) was performed until October 22, 2023, to retrieve all relevant publications. The literature review carried out with the predetermined search terms: (“SARS-CoV-2 “2019 Novel Coronavirus” OR “Coronavirus Disease 2019”) and (“COVID-19 vaccines” OR “mrna COVID-19 vaccine” OR “Pfizer” OR “moderna” OR “mRNA-1273” OR ”mRNA 1273” OR “messenger RNA vaccine” OR “ChAdOx1” OR “ChAdOx1 nCoV 19” OR “AstraZeneca, COVID-19 Vaccine”) and (“inflammatory heart disease*” OR “inflammatory cardiac disease*” OR “heart failure” OR “cardiac manifestation*” OR “stroke” OR “ischemic heart disease” OR “Coronary Artery Disease” OR “myocardial infarction” OR “arrhythmia” OR “myocardial damage”). Moreover, we thoroughly examined the references of all relevant articles to identify any additional studies meeting our criteria.
Inclusion and exclusion criteria
We included all studies on humans and focused on adverse events specifically cardiovascular events occurring after COVID-19 vaccination. Information of individuals who experienced cardiovascular events following any COVID-19 vaccine, regardless of the vaccine type or dosage were extracted. We excluded narrative and systematic reviews, case reports studies, or original papers that lacked available data. Additionally, articles written in languages other than english were excluded from the review.
Data Screening procedure
The study followed the PRISMA 2020 guidelines for data extraction, adhering to a standardized process. Two authors independently screened abstracts and full-text articles based on pre-defined inclusion and exclusion criteria, with any disagreements resolved through discussion in Table S1. Microsoft excel spreadsheets were used to collect the necessary information from the extracted studies. This included 1) essential details such as the first author, publication year and study design; 2) information of the study population, including sample sizes, age, gender, follow-up duration and locations; 3) information on COVID-19 vaccine types, number of doses administered and reported cardiovascular events in each study; and 4) Information needed for data analysis includes the frequency of cardiovascular events following COVID-19 vaccination and in the control group (unvaccinated or inactive vaccine) during the study period. The study’s outcomes centered on myocardial infarction, arrhythmia, stroke and Coronary Artery Disease (CAD) or Coronary Heart Disease (CHD). These outcomes were identified using the 10th edition of the International Classification of Diseases, as detailed in Table S2.
Quality assessment
The quality of the included articles was assessed by the two reviewers independently using two checklists. NHLBI quality assessment tools was used for case-series studies [22] and the Newcastle-Ottawa Scale assesses explicitly the quality of cohort studies [23]. The cohort tool includes eight questions and the prevalence tool includes nine questions, each scoring 0 or 1, to determine the potential flaws in study methods or implementation. The overall methodological quality judgments will be determined by the total score for each article as follows: Low quality (≤ 50% of overall score), moderate rate (50%-70% of overall score) and high quality (≥ 70% of overall score). Tables are available in Table S3 and S4.
Data synthesis and analysis
In this investigation, we examined N studies that evaluated the desired outcomes following the COVID-19 vaccine. Since the multivariate approach enables us to estimate correlations in treatment effects among studies as an integral part of a random effects model, we applied this method to combine the results. As the studies may not reported all the events we were interested in, to address this limitation, we employed multivariate normal models with different dimensions, 1 ≤ pi ≤ p. Where pi represents the number of effects reported by study i, (i=1,2,…,N).
We modeled the data as follows:
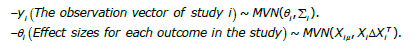
The primary goal in the multivariate random-effects meta-analysis is to estimate the mean treatment effects θ = (θ1,θ2,…,θm) and the between-study covariance matrix, Δ [24,25]. To achieve this goal, we utilized Bayesian methods and considered prior distributions for these parameters [26]. We employed three different priors, Invers-Wishart, Cholesky and Spherical for the variance-covariance matrix of the between-study, along with a multivariate normal distribution for the mean vector, μ. The inverse-Wishart prior serves as the conjugate prior distribution for the variance-covariance matrix of the between-study component in multivariate normal models [27,28].
The Cholesky parameterization allows for assumptions of homogeneity in between-study correlations, while the Spherical parameterization incorporates a prior assumption of positive between-study correlation. Subsequently, we presented the findings corresponding to the structure or prior that yielded the best overall fit for the model [24].
By running The Markov Chain Monte Carlo (MCMC) in parallel with a substantial number of iterations for each chain and including a burn-in period, the algorithm can converge to the target distribution and produce reliable results.
The convergence was evaluated using visual diagnostics for specific parameters of interest within the models. It is essential to note that we did not have information about within-study covariances. So we estimated it with methods developed by Wei and Higgins [29]. To ensure the robustness of our results, we conducted a subgroup analysis, considering factors such as dose, type of vaccine and geographical region.
Model execution
The multivariate Bayesian meta-analysis models were run using R version 4.3.2 and the “rjags” package version 4-14. The MCMC model output was summarized using the “coda” package [30]. Four parallel MCMC chains were run, each consisting of 100,000 iterations with a burn-in period of 10,000 iterations. The datasets (studies) used and analyzed during the current study are available in Tables 1 and 2 and the JAGS code for the model is provided in supplementary file.
| First author | Study design | Country | Study period | Dose Vaccine | Follow up | Age | Type of vaccine, n | Control group, n | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Carlos King Ho Wong (2022) | Retrospective cohort | Hong kong | Dec 14, 2021 to Jan 1, 2022 | Dose 1 Dose 2 |
21 days after first and second doses | >18 years old | BNT162b2: First dose:1308820 Seconded dose: 1116677 |
Inactivated vaccine: First dose:955859 Seconded dose: 821560 |
Arrhythmia CAD MI |
| Norazida Ab Rahman (2022) | Self-controlled case-series (SCCS) | Malaysia | February 1, 2021 to September 30, 2021 | Dose 1 Dose 2 |
21 days after first and second doses | >18 years old | BNT162b2: 15488664 ChAdOx1: 2816121 |
Unvaccinated: 16896724 | Arrhythmia MI Stroke |
| Maria Elena Flacco (2022) | Retrospective cohort | Italian | 2 January 2021 to 31 July 2022 | Dose 1 Dose 2 Dose 3 |
6 months after first and second doses | >6 years old | BNT162b2 First dose: 8106 Seconded dose: 34422 Third dose: 73845 mRNA-1273 First dose: 7504 Seconded dose: 8011 Third dose: 22884 Janssen Seconded dose: 1085 ChAdOx1 First dose: 190 Seconded dose: 6719 |
Unvaccinated First dose:56494 Seconded dose: 56494 |
Arrhythmia MI Stroke |
| Noam Barda (2021) | Retrospective cohort | Israel | December 20, 2020 to May 24, 2021 | Dose 1 Dose 2 | 21 days after the first or second doses | ≥ 16 years old | BNT162b2 mRNA:884828 | Unvaccinated: 884828 | Arrhythmia MI |
| Jeremie Botton (2022) | Self-controlled case-series (SCCS) | France | December, 27 2020 to July, 20 2021 | Dose 1 Dose 2 Dose 3 |
21 days after each of the first, second and third doses | 18–74 years | BNT162b2 First dose: 38393 Seconded dose: 31385 mRNA-1273 First dose: 5343 Seconded dose: 4099 Janssen First dose: 593 ChAdOx1 First dose: 8358 Seconded dose: 4887 |
Unvaccinated First dose: 20640 Seconded dose: 32947 |
MI Stroke |
| Young-Eun Kim (2022) | Retrospective cohort | Korea | July 2020 and December 2021 | Dose 1 | 84 days after vaccination | >18 years old | BNT162b2: 168 310 | Unvaccinated: 62 727 | MI Stroke |
| William N. Whiteley (2022) | Retrospective cohort | England | December 8, 2020 to March 18, 2021 | Dose 1 | 28 days after vaccination | >18 years old | BNT162b2: 8712477 ChAdOx1: 12481337 | Unvaccinated: 10563566 | MI Stroke |
| Eric Yuk Fai Wan (2022) | Self-controlled case-series | Hong kong | 23 February 2021 and 31 January 2022 | Dose 1 Dose 2 |
21 days after the first or second doses | ≥ 16 years old | BNT162b2: 141224 | Inactivated vaccine: 209739 |
Arrhythmia CAD |
| Francisco Tsz Tsun Lai (2022) | Retrospective cohort | Hong kong | to September 30, 2021 | Dose 1 Dose 2 |
28 days following the first and second doses | 12–18 years | BNT162b2 First dose:138141 Seconded dose: 119664 |
Unvaccinated: First dose:136743 Seconded dose:118300 |
Arrhythmia CAD |
| Barbara H. Bardenheier (2021) | Cohort study | US | December 18, 2020 to March 7, 2021 | Dose 1 Dose 2 |
15 days | Average age ≥ 60 years | BNT162b2 First dose:8553 Seconded dose: 8371 |
Unvaccinated: 11,072 |
MI Stroke |
| Julia Hippisley-Cox (2021) | Self-controlled case-series | England | December 20, 2020 to May 24, 2021 | Dose 1 | 28 days | ≥16 years old | BNT162b2: 19608008 | Unvaccinated: 19608008 |
MI Stroke |
| Anne M. Hause (2022) | Retrospective, observational study | US | August 31,2022–October 23, 2022 | Booster dose | 7 days | ≥12 years old | BNT162b2: 122953 mRNA-1273: 89006 |
- | Arrhythmia MI Stroke |
| Soonok Sa (2022) | Observational study | US | 14 December 2020 to 30 September 2021 | - | - | ≥18 years old | BNT162b2: 205436 mRNA-1273: 237158 |
- | MI Stroke |
| Barbra A. Dickerman (2022) | Observational study | US | January 4 2021 to September 20, 2021 | Dose 1 | 14 days after first dose & 42 days after first dose |
≥18 years old | BNT162b2: 216836 mRNA-1273: 216836 |
- | Arrhythmia MI Stroke |
| Hannah G Rosenblum (2022) | Observational study | US | December 14, 2020 to June 14, 2021 | Dose 1 Dose 2 |
7 days | ≥16 years old | BNT162b2: 167177332 mRNA-1273: 131639515 |
- | MI Stroke |
Table 1: Characteristics and outcomes of patients with cardiovascular events related to COVID-19 vaccine related to 15 last articles.
| Arrythmia | MI | CAD | Stroke | |
|---|---|---|---|---|
| Total | 1.53 (0.89-2.63) | 0.76 (0.51-1.14) | 1.70 (1.11-2.57) | 1.29 (0.87-1.93) |
| Dose | ||||
| Dose1 | 2.98 (1.41-6.32) | 1.24 (0.76-2.03) | 1.01 (0.61-1.66) | 3.40 (1.98-5.86) |
| Dose2 | 0.65 (0.33-1.29) | 3.86 (2.28-6.60) | 3.44 (1.99-5.98) | 1.35 (0.83-2.20) |
| Dose3 | - | 0.003 (0.001-0.006) | - | 0.19 (0.10-0.39) |
| Vaccination | ||||
| BNT162b2 | 1.75 (0.79-3.85) | 1.87 (1.22-2.89) | 1.64 (1.06-2.55) | 2.09 (1.36-3.21) |
| Dose1 | 2.30 (0.62-5.71) | 1.13 (0.69-1.87) | 1.07 (0.64-1.77) | 3.69 (2.13-6.37) |
| Dose2 | 1.54 (0.36-6.65) | 3.84 (2.21-6.66) | 2.98 (1.64-5.37) | 1.34 (0.81-2.21) |
| ChAdOx1 | 8.11 (3.67-17.99) | 1.11 (0.33-3.74) | - | 0.47 (0.19-1.95) |
| Dose1 | 4.89 (1.21-19.38) | 16.18 (2.46-3.08) | - | 9.37 (0.96-91.25) |
| Dose2 | 0.36 (0.12-1.03) | 3.22 (0.29-3.08) | - | 0.80(0.07-9) |
| Others | 0.96 (0.39-2.41) | 1.73 (0.72-4.18) | - | 0.50 (0.24-1.03) |
| Dose1 | 0.29 (0.03-3.04) | 1.10 (0.12-10.27) | - | 0.39 (0.04-3.73) |
| Dose2 | 0.97 (0.30-3.22) | 3.99 (1.06-15.19) | - | 1.58 (0.38-6.43) |
| Geographical location | ||||
| Asia | 2.23 (0.99-4.97) | 0.27 (0.07-1.53) | 1.60 (1.03-2.46) | 0.29 (0.05-1.83) |
| Europe | 1.36 (0.72-2.58) | 0.80 (0.54-1.20) | - | 1.33 (0.89-2.00) |
Note: MI: Myocardial Infarction; CAD: Coronary Artery Disease
Table 2: Results of Bayesian multivariate meta-analyses, subgroup analysis and Odds Ratio (95% CI).
Results
Selection of studies
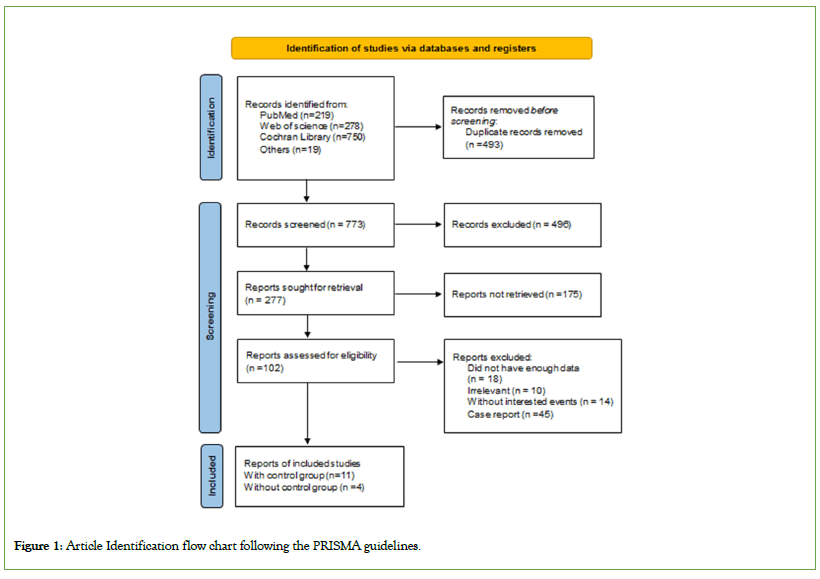
Upon searching major databases (PubMed, Web of Science, Embase, Cochrane library and Google Scholar) on 22 October, 2023, we identified 1266 articles related to search criteria. 493 studies were automatically removed due to duplicate content by utilizing Endnote as a citation manager tool. After examination of the title and abstracts of 496 articles meticulously, 175 studies were not related and did not meet our inclusion requirements. Finally, after examination of 85 remaining studies, 15 studies remained.
Out of the 15 studies, 11 were controlled studies chosen for the primary analysis, while 4 studies did not have control group and were included in the secondary analysis (Figure 1). More details about the studies can be found in Table 1. In the assessment of study quality using quality assessment tools, two out of the seven cohort studies and nine self-control case series studies reviewed were rated as medium-quality, as shown in Tables S3 and S4. The remaining studies were determined to be of high quality based on the evaluation criteria specified in the quality assessment tools. This indicates that the majority of the reviewed studies demonstrated a high level of methodological rigor and reliability in their design and execution (Figures 2-4).
Figure 1: Article Identification flow chart following the PRISMA guidelines.
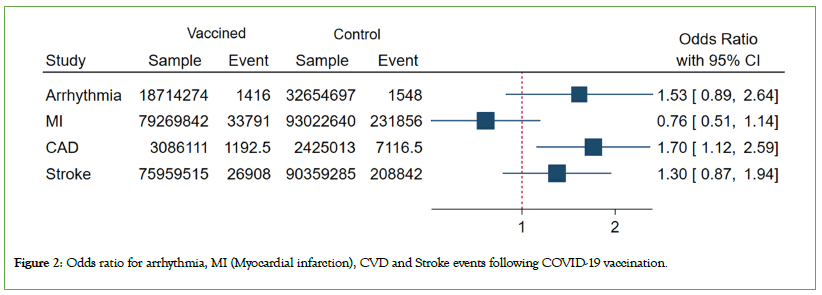
Figure 2: Odds ratio for arrhythmia, MI (Myocardial infarction), CVD and Stroke events following COVID-19 vaccination.
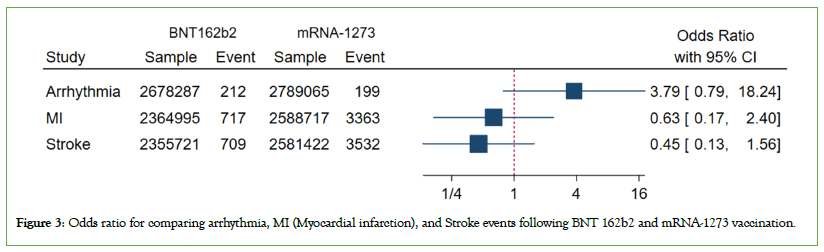
Figure 3: Odds ratio for comparing arrhythmia, MI (Myocardial infarction) and Stroke events following BNT 162b2 and mRNA-1273 vaccination.
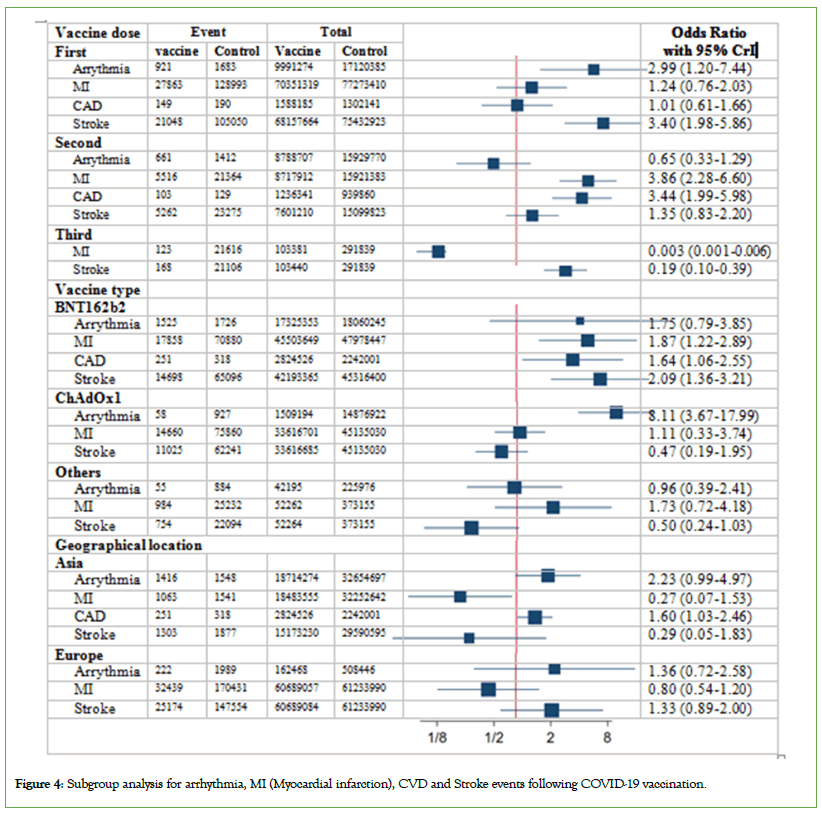
Figure 4: Subgroup analysis for arrhythmia, MI (Myocardial infarction), CVD and Stroke events following COVID-19 vaccination.
Feature of the extracted studies
Eleven studies were included in the primary analysis: four were conducted in Hong Kong, two were related to England and the remaining studies took place in the United States of America, Thailand, Israel, France and Korea. A total of 37774228 individuals received the first dose of the vaccine, 8076761 received the second dose and 199021 received the third dose. Additionally, 39898214 individuals either did not receive any vaccine or were given an inactive vaccine in the control group. Four studies analyzed the outcomes of the first and second doses of vaccine. Two studies looked at the effects of the first, second, or third doses, while the remaining studies focused on either the first dose or any dose of the vaccine. All studies analyzed the BNT162b2 vaccine, four studies looked into the ChAdOx1 and two studies investigated other vaccines in addition to BNT162b2.
In the secondary analysis, four studies from the United States of America were included, with 167722557 individuals in the BNT162b2 (Pfizer) vaccine group and 132182515 individuals in the mRNA- 1273 (Moderna) vaccine group. Age of participants in all studies was above 16 years old, except for one study, which focused on individuals aged between 12 and 18 years.
Bayesian multivariate and univariate results
Primary analysis: Based on the Bayesian multivariate metaanalysis, among the examined cardiovascular events, only CAD was notable. As evident from the findings, the overall odds of CAD events in the vaccine group exceeded than the control group (OR, 1.70; 95% CrI: 1.11–2.57). Five studies reported CAD, all of which were BNT162b2 (OR, 1.64; 95% CrI: 1.06-2.55) and from Asian countries. Moreover, examining the results by vaccine dose, we observed that the odds of CAD were not significant for the first dose (OR, 1.01; 95% CrI: 0.61–1.65), but significant for the second dose (OR, 3.44; 95% CrI: 1.99-5.98). However, no significant relationship was detected between vaccination and stroke, myocardial infarction and arrythmia.
Subgroup analyzes were conducted to further investigate these findings by considering vaccine type, doseand geographical location. Results based on vaccine type revealed a link between the BNT162b2 vaccine and an increased risk of myocardial infarction (OR, 1.87; 95% CrI: 1.22-2.89) and stroke (OR, 2.09; 95% CrI: 1.36-3.21). These findings were significant for stroke following the first dose (OR, 3.69; 95% CrI: 2.13-6.37) and for myocardial infarction after the second dose of BNT162b2 (OR, 3.84; 95% CrI: 2.21-6.66). The ChAdOx1 vaccine, in general, showed no significant association with any of the events. Just a notable link between the increased risk of arrhythmia and the ChAdOx1 was observed in relation to the first dose (OR, 4.89; 95% CrI: 1.21– 19.38).
Examining the results by dose, irrespective of the vaccine type, revealed that the first dose was linked to a higher risk of arrhythmia (OR, 2.98; 95% CrI: 1.41-6.32) and stroke (OR, 3.40; 95% CrI: 1.98-1.98). As mentioned in subgroup findings on vaccine type indicated that arrhythmia was associated with the first dose of the ChAdOx1, while stroke was associated with the first dose of BNT162b2. In contrast, the second dose exhibited a higher risk of myocardial infarction (OR, 3.86; 95% CI: 1.99–5.98) and CAD (OR, 3.44; 95% CrI: 1.99-5.98). Interestingly, the third dose had no impact on myocardial infarction (OR, 0.003; 95% CI: 0.001–0.006) and decreased the risk of stroke (OR, 0.20; 95% CI: 0.10–0.39).
Except for the case of CAD related to Asian countries, no significant findings were noted based on geographical region for any of the outcomes
Secondary analysis: To compare BNT162b2 and mRNA vaccines as a secondary objective, we merged the findings of 6 studies, all conducted in the United States of America. Among these, 4 studies compared BNT162b2 and mRNA vaccines, while 2 studies compared these vaccines with an unvaccinated group. Ultimately, upon consolidating the results of these studies, we observed no significant difference between the two vaccines regarding the odds of cardiovascular consequences.
Discussion
To the best of our knowledge, this is the first meta-analysis that represents the pioneering effort in conducting a multivariate analysis of COVID-19 vaccine-related cardiovascular events. Distinguishing our study from previous meta-analyzes, we exclusively focused on controlled observational studies, which are recognized for providing more robust evidence than case reports or non-controlled observational studies. Concentrating on controlled observational studies, we aimed to mitigate biases and confounding factors that could influence the association between the vaccines and cardiac complications. Prior systematic review and meta-analysis studies predominantly relied on case reports, case series, or a combination of these with observational or cohort studies, lacking direct comparisons with control groups [15-20]. Furthermore, the present study differs from most meta-analyzes that primarily focused on myocarditis and pericarditis as common post-vaccine cardiac side effects [16,17].
Our primary analyzes, conducted through Bayesian multivariate meta-analysis, uncovered notable insights regarding the impact of COVID-19 vaccines on cardiovascular health. Specifically, we found that the administration of COVID-19 vaccines, particularly BNT162b2, was associated with increased odds of CAD following the second dose. However, it’s important to highlight that the odds of experiencing myocardial infarction, stroke and arrhythmia did not exhibit significant elevation due to the administration of COVID-19 vaccines. Subgroup analysis revealed a significant increase in arrhythmia and stroke risk after the first vaccine dose, a rise in myocardial infarction and CVD risk post-second dose and no significant association after the third dose. Some outcomes even exhibited a protective effect, possibly due to higher stress levels during early phases of vaccination, contrasting with reduced stress and increased vaccine confidence in the third phase. Analysis by vaccine type indicated that the BNT162b2 vaccine was notably linked to increased risk for all events except arrhythmia. In contrast, the ChAdOx1 vaccine primarily affected arrhythmia risk, especially after the first dose, while other vaccines showed no significant effects [31-39].
A secondary objective of our research involved comparing the BNT162b2 vaccine with mRNA-1273 vaccine to assess any differences in their effects on cardiovascular health. To achieve this, we synthesized the findings of six independent studies, all of which were conducted in the USA. After meticulous analysis and consolidation of the results from these studies, our investigation yielded an intriguing finding. Despite variations in study methodologies and populations, there was a consistent observation: No significant difference was observed between the Pfizer BioNTech vaccine and mRNA-1273 vaccine concerning the odds of cardiovascular consequences. This implies that both types of mRNA vaccines were similarly effective or lacked substantial variance in their impact on cardiovascular health. mRNA vaccines encode the prefusion stabilized full-length spike protein of SARSCoV- 2, but they use slightly different systems for intracellular delivery. Yet, the specific mechanisms behind any observed differences in safety profiles remain unclear.
Two meta-analyzes examining the relationship between cardiovascular events and COVID-19 vaccination recently published. Study by Chang et al., published in 2023, investigated not only myocarditis but also myocardial infarction and arrhythmia [19]. The study found no significant association between COVID-19 vaccination and incidence of myocardial infarction or arrhythmia, which aligns with the findings of our research. Contrary to our study, subgroup analysis in this research did not yield significant results regarding vaccine dose or type. Similarly, Khaity et al., did not find a significant relationship between arrhythmia and the vaccine [20]. The study analyzed published cases and did not examine results based on vaccine dosage. Anyway, the consistent results of these two studies regarding arrhythmia and myocardial infarction support the findings of the multivariate model in our research. The assessment of myocardial infarction risk post COVID-19 vaccination was also examined in a systematic review conducted by Petrudi et al., their analysis of case report studies concluded that instances of myocardial infarction subsequent to COVID-19 vaccination are infrequent. Likewise, the analysis by Baqi et al., which scrutinized 10 case reports and 5 case series studies, underscored that myocardial infarction associated with COVID-19 vaccination is an uncommon yet severe and potentially life-threatening occurrence.
In terms of stroke, our multivariate results align with a previously conducted meta-analysis conducted in England using the selfcontrolled case series design [31-33] and population studies from France, the United States of America and Israel [4,34,35]. All of the studies found no increased incidence of stroke following vaccination. In contrast, a recent and comprehensive analysis conducted by Jiang in 2023 [36], revealed a 41% reduction in the risk of post-COVID heart attack or stroke among fully vaccinated individuals. In the study mentioned that, even partial vaccination was associated with a decreased risk of adverse cardiovascular events, consistent with the findings from our subgroup meta-analysis about myocardial infarction and stroke after third dose.
To compare our findings on CAD, we have not come across any research examining the connection between CAD and the corona vaccine. The results from this study consist of 5 studies, all focusing on the BNT162b2 vaccine and in Asia, indicating a need for further research and exploration in this area.
The concerns regarding a potential link between adverse cardiovascular events and COVID-19 vaccines have prompted various hypotheses to explain the underlying mechanism, although the exact pathogenesis remains unclear. One hypothesis suggests a correlation between vaccine-induced immune syndrome and CVD [37]. One of the particular concern is the autoimmune reaction following vaccination, especially for individuals with a complex medical history [38]. This is because the immune system plays an important role in both cardiac composition and function, which can potentially trigger an excessive immune response in certain individuals, leading to autoimmune cardiac injury [39]. Additionally, the immune system has various effects on ischemic injuries, such as MI and ischemic stroke, involving both innate and adaptive immune cells [37]. Proposed mechanisms for COVID-19 vaccine-induced myocardial infarction may be attributed to Vaccine- Induced Thrombotic Thrombocytopenia (VITT), a condition akin to heparin-induced thrombocytopenia [40,41]. Another hypothesis posits that following vaccination, there may be a mismatch between the supply and demand of oxygen in a cardiovascular system already affected by disease [42]. Additionally, there is a possibility that COVID-19 vaccines may trigger a vasospastic allergic myocardial infarction, a condition known as Kounis syndrome [43,44].
Overall, our study contributes to the existing literature by employing a comprehensive analysis approach and emphasizing controlled observational studies. While acknowledging potential side effects, our findings support the overall safety of the COVID-19 vaccine concerning cardiovascular complications such as myocardial infarction, stroke and arrhythmia. However, it is important to note that ongoing surveillance and research are essential to continually monitor the safety and efficacy profiles of vaccines, including their potential cardiovascular effects, particularly as new variants emerge and vaccination strategies evolve. This underscores the importance of robust and continuous post-marketing surveillance systems to promptly identify and address any emerging safety concerns associated with vaccines.
Conclusion
This is the first meta-analysis focusing on COVID-19 vaccinerelated cardiovascular events in controlled observational studies, aiming to reduce biases. The study found BNT162b2 linked to increased CAD risk after the second dose. Various risks were analyzed post-vaccination doses, with different impacts based on vaccine type. Comparison between BNT162b2 and mRNA- 1273 vaccines showed no significant difference in cardiovascular effects. The findings of the present study may help public health policy for future pandemics, consider CAD in the context of COVID-19 vaccination and assess the cardiac condition before the choice of vaccine is offered to adults. To minimize such risks, it is recommended that comprehensive pre-clinical and clinical studies be conducted to assess the cardiovascular safety of new vaccines, including large-scale trials involving diverse populations.
Limitations
Among the limitations of our study, one noteworthy factor is the limited number of included studies. This restriction arises from the scarcity of studies available in the field that possess a control group. Consequently, due to the small sample sizes within subgroups, specific subgroup analyzes could not be conducted. In addition, the absence of reported data on the 3rd dose of the vaccine, except for just 2 studies, prohibited further analyzes related to this aspect. Furthermore, to gain a more comprehensive understanding, future investigations should encompass age and gender subgroups. Nevertheless, the potential impact of this discrepancy on the precision of the findings may be minimal.
Conflict of interest
The authors state that they have no conflicts of interest to disclose. They also confirm that all authors were involved in preparing the manuscript and have reviewed and approved the final version submitted for publication.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
References
- WHO Coronavirus (COVID-19) Dashboard. 2023.
- Ritchie H, Mathieu E, Rodés GL, Appel C, Giattino C, et al. Coronavirus pandemic (COVID-19). Published online at OurWorldInData.
- World Health Organization (2022) WHO COVID-19 Dashboard.2022.
- Barda N, Dagan N, Ben SY, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021; 385(12):1078-90.
[Google Scholar] [PubMed]
- Rosenblum HG. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the Advisory Committee on Immunization Practices—United States of America, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70.
[Crossref][Google Scholar] [PubMed]
- Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-6.
[Crossref] [Google Scholar] [PubMed]
- Hippisley C J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study.BMJ. 2021;374.
[Crossref] [Google Scholar] [PubMed]
- Lai FT, Chua GT, Chan EW, Huang L, Kwan MY, Ma T, et al. Adverse events of special interest following the use of BNT162b2 in adolescents: a population-based retrospective cohort study. Emerg Microbes Infect. 2022;11(1):885-93.
[Crossref] [Google Scholar] [PubMed]
- Kim YE, Huh K, Park YJ, Peck KR, Jung J. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. 2022; 328(9):887-9.
[Crossref] [Google Scholar] [PubMed]
- Ye X, Yan VK, Yiu HH, Shami JJ, Kang W, Ma T, et al. BNT162b2 or CoronaVac vaccinations are associated with a lower risk of myocardial infarction and stroke after SARSâ?CoVâ?2 infection among patients with cardiovascular disease. J Am Heart Assoc. 2023;12(9):e029291.
[Crossref] [Google Scholar] [PubMed]
- Whiteley WN, Ip S, Cooper JA, Bolton T, Keene S, Walker V, et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 2022;19(2): e1003926.
[Crossref] [Google Scholar] [PubMed]
- Wong CK, Lau KT, Xiong X, Au IC, Lai FT, Wan EY, et al. Adverse events of special interest and mortality following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines in Hong Kong: A retrospective study. PLoS Med. 2022;19(6):e1004018.
[Crossref] [Google Scholar] [PubMed]
- Botton J, Jabagi MJ, Bertrand M, Baricault B, Drouin J, Le Vu S, et al. Risk for myocardial infarction, strokeand pulmonary embolism following COVID-19 vaccines in adults younger than 75 years in France. Ann Internal Med. 2022;175(9):1250-7.
[Crossref] [Google Scholar] [PubMed]
- Wan EY, Chui CS, Mok AH, Xu W, Yan VK, Lai FT, et al. mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination and risk of adverse events and acute diabetic complications in patients with type 2 diabetes mellitus: a population-based study. Drug Saf. 2022 ;45(12):1477-90.
[Crossref] [Google Scholar] [PubMed]
- Ahmed SK, Mohamed MG, Essa RA, Rashad EA, Ibrahim PK, Khdir AA, et al. Global reports of myocarditis following COVID-19 vaccination: A systematic review and meta-analysis. Diabetes Metab Syndr. 2022;16(6):102513.
[Crossref] [Google Scholar] [PubMed]
- Chou OH, Mui J, Chung CT, Radford D, Ranjithkumar S, Evbayekha E, et al. COVID-19 vaccination and carditis in children and adolescents: a systematic review and meta-analysis. Clin Res Cardiol. 2022;111(10):1161-73.
[Crossref] [Google Scholar] [PubMed]
- Oueijan RI, Hill OR, Ahiawodzi PD, Fasinu PS, Thompson DK. Rare Heterogeneous Adverse Events Associated with mRNA-Based COVID-19 Vaccines: A Systematic Review. Medicines. 2022;9(8):43.
[Crossref] [Google Scholar] [PubMed]
- Ling RR, Ramanathan K, Tan FL, Tai BC, Somani J, Fisher D, et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. The Lancet Respir Med. 2022;10(7):679-88.
[Crossref] [Google Scholar] [PubMed]
- Chang Y, Lv G, Liu C, Huang E, Luo B. Cardiovascular safety of COVID-19 vaccines in real-world studies: a systematic review and meta-analysis. Expert Rev Vaccines. 2023;22(1):25-34.
[Crossref] [Google Scholar] [PubMed]
- Khaity A, Abdelwahab OA, Albakri K, Diab RA, Al-DNM, Abbassy M, et al. Cardiovascular disease and COVID-19 vaccines: a systematic review and analysis of published cases. Eur Cardiol. 2023;18.
[Crossref] [Google Scholar] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372.
[Crossref] [Google Scholar] [PubMed]
- Background: Development and Use of Study Quality Assessment Tools.
- Wells GA, Shea B, Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- Nam IS, Mengersen K, Garthwaite P. Multivariate metaâ?analysis. Stat Med. 2003;22(14):2309-33.
[Crossref] [Google Scholar] [PubMed]
- Van HHC, Arends LR, Stijnen T. Advanced methods in metaâ?analysis: multivariate approach and metaâ?regression. Stat Med. 2002;21(4):589-624.
[Crossref] [Google Scholar] [PubMed]
- Wei Y, Higgins JP. Bayesian multivariate metaâ?analysis with multiple outcomes. Stat Med. 2013;32(17):2911-34.
[Crossref] [Google Scholar] [PubMed]
- Daniels MJ, Pourahmadi M. Bayesian analysis of covariance matrices and dynamic models for longitudinal data. Biometrika. 2002;89(3):553-66.
- Schervish MJ. Theory of statistics. Springer Science & Business Media. 2012.
[Crossref]
- Wei Y, Higgins JP. Estimating withinâ?study covariances in multivariate metaâ?analysis with multiple outcomes. Stat Med. 2013;32(7):1191-205.
[Google Scholar] [PubMed]
- Plummer M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. InProceedings of the 3rd international workshop on distributed statistical computing. 2003; 124 :1-10.
- Stefanou MI, Palaiodimou L, Aguiar de SD, Theodorou A, Bakola E, Katsaros DE, et al. Acute arterial ischemic stroke following COVID-19 vaccination: a systematic review and meta-analysis. Neurology. 2022; 99(14):e1465-74.
- Patone M, Handunnetthi L, Saatci D, Pan J, Katikireddi SV, Razvi S, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144-53.
[Crossref] [Google Scholar] [PubMed]
- Hippisley CJ, Patone M, Mei XW, Saatci D, Dixon S, Khunti Ket al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374.
[Crossref] [Google Scholar] [PubMed]
- Jabagi MJ, Botton J, Bertrand M, Weill A, Farrington P, Zureik M, et al. Myocardial infarction, strokeand pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA. 2022;327(1):80-2.
[Crossref] [Google Scholar] [PubMed]
- Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390-9.
[Crossref] [Google Scholar] [PubMed]
- Jiang J, Chan L, Kauffman J, Narula J, Charney AW, Oh W, et al. Impact of vaccination on major adverse cardiovascular events in patients with COVID-19 infection. J Am Coll Cardiol. 2023;81(9):928-30.
[Crossref] [Google Scholar] [PubMed]
- Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Imm. 2018;18(12):733-44.
[Crossref] [Google Scholar] [PubMed]
- Cines DB, Bussel JB. SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. N Eng J M. 2021;384(23):2254-6.
[Crossref] [Google Scholar] [PubMed]
- Chamling B, Vehof V, Drakos S, Weil M, Stalling P, Vahlhaus C, et al. Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis?. Clin Res Cardiol. 2021; 110:1850-4.
[Crossref] [Google Scholar] [PubMed]
- Baronti A, Gentile F, Manetti AC, Scatena A, Pellegrini S, Pucci A, et al. Myocardial infarction following COVID-19 vaccine administration: post hoc, ergo propter hoc?. Viruses. 2022;14(8):1644.
[Crossref] [Google Scholar] [PubMed]
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle P, Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination. R R Inf Dis. 2021.
- Boivin Z, Martin J. Untimely myocardial infarction or COVID-19 vaccine side effect. Cureus. 2021;13(3).
[Crossref] [Google Scholar] [PubMed]
- Kounis NG, Mazarakis A, Tsigkas G, Giannopoulos S, Goudevenos J. Kounis syndrome: a new twist on an old disease. Future Cardiol. 2011;7(6):805-24.
[Crossref] [Google Scholar] [PubMed]
- Kounis NG, Koniari I, de Gregorio C, Velissaris D, Petalas K, Brinia A, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causalityand therapeutic considerations. Vaccines. 2021;9(3):221.
[Crossref] [Google Scholar] [PubMed]
Citation: Karimi R, Norozirad M, Esmaeili F, Mansourian M (2024). Cardiovascular Events Post COVID-19 Vaccination: A Systematic Review and Bayesian Multivariate Meta-Analysis of Observational Studies. J Vaccines Vaccin. 15:559.
Copyright: © 2024 Karimi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.