Indexed In
- Academic Keys
- ResearchBible
- CiteFactor
- Access to Global Online Research in Agriculture (AGORA)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 10, Issue 3
Breeding for Drought Tolerance by Monitoring Chlorophyll Content
Mariela Inés Monteoliva1,2,3,4*, María Carla Guzzo1 and Gisella Anabel Posada52Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Córdoba, Argentina
3Department of Microbiology, Oklahoma University (OU), Córdoba, Argentina
4Department of Plants Physiology, Washington State University (WSU), Córdoba, Argentina
5Instituto of Sabin, Córdoba, Argentina
Received: 04-May-2021 Published: 24-May-2021, DOI: 10.35248/2329-6682.21.10.165
Abstract
Crop yields have increased substantially during the last 50 years, but the traits that drove these remarkable improvements, such as plant architecture, have a little remaining potential for improvement. New traits such as photosynthesis, as the ultimate determinant of yield, must be explored to support future demands. However, improving photosynthetic efficiency has played only a minor role in improving crop yield to date. Chlorophylls are the pigments allowing light to be transformed into carbohydrates, and therefore help to maintain crop yield under stress. Chlorophyll content correlates with higher yields in diverse conditions. In this review, we discuss using chlorophyll content as the basis of screens for drought tolerance. We review chlorophyll-related responses to drought in different plants and summarize the advantages and disadvantages of current methods to measure chlorophyll content, with the ultimate goal of improving the efficiency of crop breeding for drought tolerance
Keywords
Chlorophyll; Photosynthetically; Oxygenases; Pheophytin
Introduction
The unrelenting growth of the global population leads to an ever-increasing demand for food. The average yield of the main crops (such as wheat, maize, rice, and soybean) has increased substantially in the last 50 years [1], supporting The Green Revolution. However, the traits that drove the remarkable yield increases, such as optimization of shoot architecture and biomass partition to harvested product, appear to have a little remaining potential for further improvement [2]. Therefore, new and previously unappreciated traits should be (re)evaluated for their potential contribution to crop yield.
Plant biomass is largely derived from photosynthetically captured carbon. Variations in the efficiency of photosynthesis can lead to variations in growth rate and productivity, which are important factors in crop yield [3]. Directly improving photosynthetic efficiency has played a minor role in increasing yield to date, most likely due to the observation that yield was more limited by the strength of the sinks than by photosynthetic capacity itself [4-6]. Then the yield is limited by the ability to transport the carbon products to the grain more than the light interception capacity and carbon fixation rate. However, more recently, researchers have demonstrated that increased rates of photosynthesis can lead to higher yields in soybean and rice, grown under high CO2 concentrations [6-8], supporting the possibility to increase photosynthesis efficiency even more in those crops. Additionally, it is worth mentioning that other crops still have a large potential to improve yields by the optimization of light interception capacity, the conversion efficiency to biomass, and carbon partition to harvested products [2,6].
Plant stress, such as drought, negatively impacts plant growth and yield. For instance, between 1983 and 2009, global harvested areas for rice, maize, rice, and soybean were affected by drought in a 75, 82, 62, and 91% (respectively) [9], and researches estimate further reductions of crop yield in the next century (around 10- 20% in case of moderate to severe droughts for the same crops) [10]. In the antenna complex of the chloroplasts, chlorophylls are the critical pigments that capture the light to be transformed into carbohydrates during photosynthesis. The use of chlorophyll content as a trait may contribute to increase light interception and efficiency of conversion, and therefore to maintain/increase crop yield under stress [11,12]. Photosynthesis-related traits, and particularly chlorophyll content, have been studied for decades, but they have been under-utilized as traits to screen genotypes for drought tolerance. However, a myriad of studies has found that chlorophyll content is reduced in response to drought and that chlorophyll maintenance correlates with drought tolerance; however, only a few of them explicitly calculate the correlation between the chlorophyll content with yield under drought [13-38] (Table 1). Here we propose that screening for chlorophyll content is a simple and effective strategy to identify drought-tolerant crops, to complement other traits.
Chemical and Physical Properties of the Chlorophylls
The predominant chlorophylls found in photosynthetic tissues of higher plants are reduced porphyrins (dihydro porphyrins) that contain a centrally bound Mg2+ ion and are linked to a long hydrophobic phytol tail through the esterification of the acid group at C-17 [39,40] (Figure 1). During photosynthesis, antenna pigments in chloroplasts absorb solar radiation, and the resulting excitation is directed to the reaction center pigments, which release electrons and initiate the photochemical process [41,42]. Chlorophyll absorbs strongly in the red (650-700 nm) and blue (400-500 nm) regions, although absorption in the blue by carotenoids often prevents this region from being useful in chlorophyll estimation. The most abundant chlorophylls are types a and b, which absorb most of the light on the two bands of the visible spectrum in a complementary way: chlorophyll a absorbs more in the red-orange band (640-670 nm), whereas chlorophyll b absorbs more in the blue-purple band (430-460 nm). Besides, the presence of a methyl group in chlorophyll a instead of an aldehyde group in chlorophyll b shifts the absorption peaks in the red (669 nm vs. 644 nm) and blue (432 nm vs. 455 nm) [39], which may allow for discrimination between these two types of chlorophylls. Both chlorophyll types have a minimum of around 550 nm, which makes plants look green.
Leaf total-chlorophyll content (chlorophyll a+b) positively correlates with the amount of solar radiation absorbed by the leaves [43] and with the photosynthetic rate [44,45]. Additionally, chlorophyll content also correlates well with nitrogen content in a wide range of plant species, including crops [46-50]. Indeed, about 75% of the total nitrogen is found in chloroplasts [51]. These correlations reinforce the idea that greener plants will have higher photosynthesis rates [52], and in turn, yield.
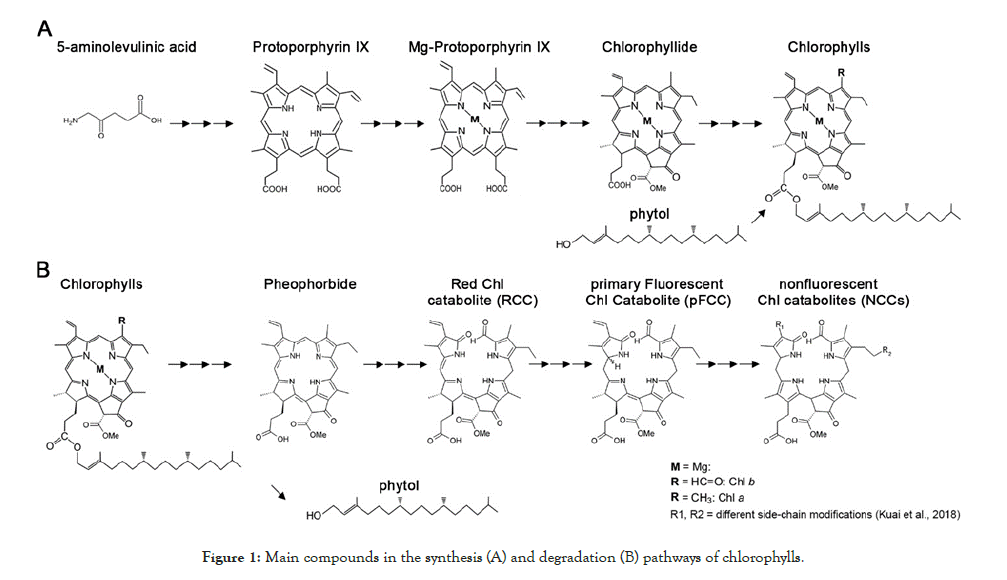
The steady-state level of chlorophylls is determined by the relative rates of anabolism and catabolism in chloroplasts (reviewed in [53] Figure 1. Chlorophylls are synthesized via the magnesium (Mg) branch of tetrapyrrole biosynthesis [40,54,55]. The first step is the formation of 5-aminolevulinic acid. Six enzymes subsequently convert eight molecules of 5-aminolevulinic acid into the precursor for chlorophylls, protoporphyrin IX. An Mg-chelatase catalyzes the insertion of Mg2+ into protoporphyrin IX, followed by a series of reactions that generate chlorophyllide. Chlorophyll synthase esterifies chlorophyllide to form the hydrophobic chlorophyll a, which can be further converted into chlorophyll b by chlorophyllide a oxygenase. Finally, newly synthesized chlorophylls a and b are rapidly integrated into the chlorophyll-binding proteins of light-harvesting chlorophyll a/b–protein complexes [56].
Figure 1: Main compounds in the synthesis (A) and degradation (B) pathways of chlorophylls.
On the other hand, chlorophyll breakdown is initiated by conversion of chlorophyll b into chlorophyll a by reductases and oxygenases in the so-called “chlorophyll cycle” (reviewed by [57,58]). Mg2+ is removed from chlorophyll a by a dechelatase to form pheophytin a. The phytol in pheophytin a is hydrolyzed to generate pheophorbide a, and the porphyrin ring is then cleaved to generate a series of fluorescent compounds (such as the red chlorophyll catabolite, RCC, and the primary fluorescent chlorophyll catabolite, pFCC). These compounds are finally isomerized in the vacuole into non-fluorescent products (nonfluorescent chlorophyll catabolites, or NCCs) [59-61].
Based on [55,57,61-64]. Chl, chlorophylls; M, Magnesium; Me, methyl; R, side-chain modifications.
Chlorophyll degradation predominates during leaf senescence and fruit ripening and is induced by drought and other stress conditions. The dynamics of chlorophyll turnover influence plant growth and productivity reducing the light interception capacity, but also enabling nutrient remobilization during leaf senescence. In addition, free chlorophylls and their metabolic intermediates generate singlet oxygen and toxic radicals upon illumination, which compromise tissue integrity [65]. Therefore, chlorophyll degradation is important to detoxify free chlorophylls [53,66]. However, protecting chlorophyll from degradation induced by stress (such as oxidative stress) might support drought tolerance [67].
Maintaining Chlorophyll Content Predicts Drought Tolerance
In most plant species, water deficit due to drought induces the degradation of the thylakoid membrane within chloroplasts, negatively affecting chlorophyll and other photosynthetic pigments (reviewed by [68]). As a consequence of the stress, the photosynthetic rate, leaf area, and ultimately yield, are reduced [69,70]. Drought generally leads to lower total chlorophyll content and an altered proportion of chlorophyll a/b. Increased degradation of chlorophyll a under drought led to a reduction of the chlorophyll a/b ratio in barley [28,71] and in Vigna radiata [72].
However, not all plants under drought reduce their chlorophyll content (Table 1). Some drought-tolerant grasses maintain their chlorophyll content under drought [35,73], and this response has also been reported in soybean (ADM 5009 RG, [24]) and potato (UNICA, [74]). The ability to maintain chlorophyll content may vary with the genotype, and stress duration and intensity. Overall, plants that maintain a relatively higher chlorophyll content under drought use light energy more efficiently [75], which indicates higher drought tolerance.
| Family | Species | Common name | Water deficit treatment | Chlorophyll determination method | Chlorophyll content response to drought | Chlorophylls vs. yield correlation | Reference |
|---|---|---|---|---|---|---|---|
| Amaranthaceae | Beta vulgaris subsp. vulgaris var. altissima | Sugar beet | Field, supplemental irrigation for controls | Handheld meter | Reduction, less reduction in tolerant genotypes | n.d. | 13 |
| Apiaceae | Daucus carota L. | Carrot | Greenhouse, controlled irrigation reduction | Liquid extract | Reduction of total content and ratio a/b, less reduction in tolerant genotypes | n.d. | 14 |
| Arecaceae | Elaeis guineensis | Oil palm | Greenhouse, water withhold | Liquid extract | Reduction of total content and ratio a/b | n.d. | 15 |
| Asteraceae | Helianthus tuberosus L. | Artichoke | Greenhouse, water withhold | Handheld meter | Increase | n.d. | 16 |
| Brassicaceae | Brassica napus | Rapeseed | Greenhouse, water withhold | Handheld meter | Reduction | yes, n.d. | 17 |
| Brassica campestris, B. carinata, B. juncea, and B. napus | Sarson, Ethiopian mustard, Brown mustard, and Oilseed rape, respectively | Greenhouse, water withhold | Liquid extract | Reduction, less reduction in tolerant genotypes | n.d. | 18 | |
| Cucurbitaceae | Cucumis sativus L. cv. Jinyou No.1 | Cucumber | Greenhouse, PEG treatment, hydroponics | Handheld meter | Reduction | n.d. | 19 |
| C. lanatus var. lanatus | Watermelon, drought-sensitive genotype Y34 | Greenhouse, controlled irrigation reduction | Liquid extract | Reduction | n.d. | 20 | |
| Fabaceae | Arachis hypogaea | Peanut | Field, controlled irrigation reduction | Liquid extract and Handheld meter | Reduction of total content and increase of chlorophyll density | n.d. | 21 |
| Field, controlled irrigation reduction | Handheld meter | n.d. | r2=0.43, for seed size | 22 | |||
| Phaseolus vulgaris | Common bean accessions | Growth chamber, water withhold | Handheld meter | No reduction in the tolerant and reduction in the sensitive genotype | n.d. | 23 | |
| Glycine max Merr. | Soybean accessions and cultivars | Greenhouse, controlled irrigation reduction | Liquid extract | No reduction in the tolerant and reduction in the sensitive genotypes | n.d. | 24 | |
| Glycine max Merr. cv. Gongxuan | Soybean | Greenhouse, water withhold | Liquid extract | Reduction of a and a/b ratio | n.d. | 25 | |
| Cicer arietinum | Chickpea | Field, supplemental irrigation for controls | Handheld meter | n.d. | r2=0.34/0.32 (2005/2006) | 26 | |
| Lens culinaris ssp.culinaris Medikus | Lentil | Field, | Handheld meter | n.d. | r2=0.30 for seed weight and number per plant | 27 | |
| Poaceae | Hordeum vulgare L. cvv. | Barley | Greenhouse, controlled irrigation reduction | Handheld meter | No or less reduction in the tolerant and higher reduction in the sensitive genotypes | r2=0.67 | 28 |
| Greenhouse | Handheld meter | Increase in most of the genotypes | n.d. | 29 | |||
| Triticum aestivum L. | Bread wheat | Field, supplemental irrigation for controls | Handheld meter | No reduction to reduction, according to the genotype | r2=0.54 | 30 | |
| Field, supplemental irrigation for controls | Liquid extract | Reduction of total content and a/b ratio | r2=0.843 | 31 | |||
| Triticum durum Desf. | Durum wheat | Field, supplemental irrigation for controls | Handheld meter | No reduction to reduction, according to the genotype | n.d. | 32 | |
| Field, supplemental irrigation for controls | Handheld meter | n.d. | r2=0.39 | 33 | |||
| Zea mays | Corn | Field, supplemental irrigation for controls | Handheld meter | No reduction in the tolerant and higher reduction in the sensitive genotypes | n.d. | 34 | |
| Growth chamber, controlled irrigation reduction | Liquid extract | Increase | n.d. | 35 | |||
| Sorghum bicolor | Sorghum | Field, supplemental irrigation for controls | Handheld meter | Reduction | r2=0.64 | 36 | |
| Saccharum spp. | Sugarcane | Field, controlled irrigation reduction | Handheld meter | No reduction in the tolerant and higher reduction in the sensitive genotypes | r2=0.36-0.33 | 37 | |
| Solanaceae | Solanum tuberosum var. Désirée, Unica, and Sarnav | Potato | Greenhouse, controlled irrigation reduction | Handheld meter | Increase | n.d. | 38 |
*n.d., not determined or informed by the original report
Table 1: Chlorophyll content in crops under drought stress. Handheld and liquid extracts measurements correspond to SPAD and spectrophotometer unless specified.
Interestingly, the chlorophyll a/b ratio decreases during leaf senescence [67]. Senescence is induced in response to drought and might in turn trigger chlorophyll degradation. Delayed-senescence crops, also called “stay-green” crops [76], have been screened in an effort to increase yield by delaying the normal senescence process and prolonging photosynthesis [77,78]. The development of staygreen genotypes has contributed to increased yield under stressful conditions in grasses, such as wheat, maize, rice, sorghum, and barley [71,79-84] and in legumes, such as soybean [85], among other crops. However, senescence can be delayed by diverse mechanisms, such as high cytokinin levels, low ethylene perception, and better maintenance of water intake. Thus, stay-green crops may have a “functional” phenotype with a high photosynthesis rate or a “cosmetic” phenotype with reduced photosynthesis but high levels of non-functional pigmented catabolites [80,81]. Therefore, monitoring chlorophyll content alone may not ensure drought tolerance, but this limitation could be overcome by examining the photosynthesis rate of droughttolerant candidates.
In some cases, delayed senescence and chlorophyll maintenance might not improve the yield under stress [86] For example, the drought tolerance of some wheat cultivars depends on the remobilization of carbon from stems to fill the grain, prioritizing grain fill over plant cycle length. In these cases, the stay-green phenotype should be avoided because it delays or inhibits the ability to use the vegetative biomass to fill the grains [86], and does not result in higher yield under drought. We infer that the delay of senescence and chlorophyll degradation can be a tolerance response, depending on the genotype. These observations reinforce the idea that screening for chlorophyll content under specific conditions (such as under drought) could significantly contribute to breeding programs.
Overall, diverse mechanisms could protect the chlorophylls from degradation under drought. A priori, a relatively higher chlorophyll content under stress may directly cause higher photosynthesis rate and yield, or it could be the result of another mechanism (such as the activation of the antioxidant system). Then the higher (or stressinsensitive) chlorophyll content would be correlated with, but not causing, the tolerance. However, a high correlation exists for some species and we support it can be used to easily identify drought tolerance.
Methods to Quantify Chlorophylls
Most of the quantification methods are based on chlorophylls’ ability to reflect or absorb light. A decrease in chlorophyll content reduces the absorbance and, concomitantly, enhances the reflectance in the visible and infrared bands. Four of the most frequently used methods are described in Table 2.
| Method | Spectrophotometric | HPLC | Handheld meters | Remote sensors |
|---|---|---|---|---|
| Principle | Absorbance | Separation based on the interaction with the fixed and mobile phases, plus absorbance | Absorbance | Spectral reflectance |
| Sampling method | Destructive | Destructive | Non-destructive (require intact tissue) | Non-destructive |
| Accuracy | High | High | Intermediate | Low |
| Scale | Sub-leaf to the individual or pooled leaf level | Sub-leaf to the individual or pooled leaf level | Sub-leaf to the individual leaf level | Fraction of individual leaf to canopy |
| Time and cost per sample | High | High | Intermediate | Low to very low |
| Training investment | Small | Intermediate/Large | Small | Large |
| Other characteristics | Possible non-linear correlation with Chl content | Several algorithms available | ||
| Disadvantages | Affected by leaf structure | Affected by leaf structure, environment, and surroundings |
Table 2: Common methods to determine chlorophyll content in crops.
| Family | Species | Common name | Experimental condition | Determination method | Function type | Reference |
|---|---|---|---|---|---|---|
| Asteraceae | Lactuca sativa cv. Lores | Butterhead lettuce | Greenhouse, no treatment | Handheld meter/liquid extract | Linear, r²=0.92 | 91 |
| Lactuca sativa L. var. longifolia | Romaine lettuce ‘Green Star’ | Field, in tunnels, no treatment | Handheld meter/liquid extract | Linear, r²=0.97 | 92 | |
| Brassicaceae | Raphanus sativus | Radish | Greenhouse, no treatment | Handheld meter (CL-1)/liquid extract | Linear function, r²=0.65 | 93 |
| Cucurbitaceae | Cucumis sativus | Cucumber | Greenhouse, no treatment | Handheld meter (CL-1)/liquid extract | Linear function, r²=0.64 | |
| Fabaceae | Arachis hypogaea | Peanut | Field, limited irrigation | Handheld meter/liquid extract (Chl density) | Linear, r²=0.76/0.94/0.96 (at 20/40/60 days after emergence, respectively) | 21 |
| Glycine max Merr. | Soybean accessions | Field, no treatment | Handheld meter/liquid extract | Linear/power, r²=0.36/0.93 | 94 | |
| Soybean RILs | Field, no treatment (different developmental stages and parts of the canopy) | Handheld meter/liquid extract | Linear, r²=0.57-0.87 | 62 | ||
| Poaceae | Oryza sativa L. japonica var. Koshihikari | Rice | Field, N fertilization | Spectral reflectance/liquid extract | Linear function, r²=0.947 | 95 |
| Oryza sativa / Triticum aestivum | Rice/wheat | n.d. | Handheld meter/liquid extract | Power/linear, r2=0.97/0.93 | 96 | |
| Sorghum bicolor | Sorghum | Field, limited irrigation | Handheld meter/liquid extract | Linear, r²=0.91 | 97 | |
| Triticum aestivum L. cv. Isengrain | Winter wheat | Field, N fertilization | Handheld meter/liquid extract | Power, r2=0.91 | 98 | |
| Triticum aestivum / Zea mays | Wheat/Maize | Greenhouse/field and greenhouse | Handheld meter (SPAD-CCR-Dualex)/liquid extract | Homographic / exponential/linear, r2=0.94/0.91/0.96 respectively | 69 | |
| Zea mays | Corn | Greenhouse, no treatment | Handheld meter (CL-1)/liquid extract | Linear function, r²=0.74 | 99 | |
| Poaceae / Fabaceae | Zea mays / Glycine max Merr. | Maize / Soybean | Field, no treatment | Handheld meter/liquid extract | Exponential, r²=0.94 | 100 |
| Solanaceae | Licopersicon esculentum | Tomato | Greenhouse, no treatment | Handheld meter/liquid extract | Exponential/linear, r²=0.75/0.74 | 101 |
| Nicotiana tabacum L. cv. Samsun | Tobacco | Growth chamber, blue light (chloroplast movement induction) | Handheld meter/liquid extract | Cubic, r²=0.86 | 102 | |
| Solanum tuberosum cv. Bintje | Potato | Field, open-top-chamber, high CO2 | Handheld meter/liquid extract | Quadratic, r²=0.95 | 103 | |
| Field, no treatment | Handheld meter/liquid extract | Exponential, r²=0.58 | 66 | |||
| Theaceae | Camellia sinensis L. cv. Jiukeng | Tea | Field, no treatment | Handheld meter/liquid extract | Exponential, r²=0.84-0.88 | 104 |
| Rosaceae | Fragaria sp. | Strawberry | n.d. | Handheld meter/liquid extract | Linear, r²=0.92 | 105 |
| Green leaf species | Brassica oleracea var. alboglabra Bailey, Brassica rapa L. var. parachinensis, Amaranthus spinosus L., Ipomoea aquatica Forsk, Manihot utilissima Pohl, Lactuca sativaL., Brassica rapa L.subsp. chinensis, Ocimum citriodorum Vis., Lactuca sativa L. var. augustana, Brassica oleraceaL. var. capitata | Chinese kale, Chinese flowering cabbage, spinach, water spinach, cassava, green leaf lettuce, Chinese cabbage pak-choi, basil, sword-leaf lettuce, head cabbage, respectively | n.d. | Handheld meter/liquid extract (HPLC) | Linear, r²=0.74 (all species together) | 106 |
| Various crop species | O. sativa L. japonica, T. aestivum L., Z. mayz L., G. max (L.) Merr., B.vulgaris L. | Rice, wheat, corn, soybean, sugarbeet | Field, no treatment | Spectral reflectance (Ratio Spectral Index)/liquid extract | Linear function, r²=0.89 | 95 |
*n.d. not determined or reported in the text or supplemental material
Table 3: Correlations between chlorophyll content measured by handheld meters and liquid extract methods among crops under different experimental conditions. Handheld and liquid extracts measurements correspond to SPAD and spectrophotometer unless specified.
Both spectrophotometry and High-Performance Liquid Chromatography (HPLC) destroy the sample, impeding monitoring responses in the same leaf over time. Also, removing or injuring a leaf may substantially affect plant performance under certain conditions, such as during seedling establishment or under severe stress. Both methods involve chlorophyll extraction by a solvent, the determination of absorbance by the chlorophyll solution, and conversion from absorbance to concentration using standard formulas [12,74]. These methods can discriminate between a and b chlorophyll, and therefore can be used to calculate the a/b ratio. Different solvents (such as acetone, methanol, or ethanol) vary in their ability to extract pigments and alter the absorption peaks of both a and b chlorophylls. Increasing polarity and/or water content will shift the absorption peaks to longer wavelengths [51]. Spectrophotometry is the simpler and more accessible method, whereas HPLC is more accurate but expensive. Chlorophylls can also be measured by other analytical methods, such as Nuclear Magnetic Resonance, with even higher precision and cost. Overall, these destructive methods are not amenable to large-scale experiments and natural community studies [28,41,87].
Handheld meters, such as SPAD-501/2 (Minolta), CL-01 (Hansatech), CCM-200 (Optiscience), and Dualex (Force-A) [41], measure the absorbance of the chlorophylls in situ, without destroying the sample. Actually, they require intact tissue, which limits the possibility to pool samples. They measure the chlorophyll absorbance in the red peak (~620-650 nm, with a higher contribution from chlorophyll a). Also, a reference wavelength in the near-infrared (~760 nm or ~940-950 nm, according to the instrument) is used to determine the contribution of leaf structures such as cell walls [41,67,88]. However, some of these leaf structures, such as vein distribution, leaf anatomy, or water content, do not scale linearly and therefore chlorophyll quantification must be validated with an extractive method [21,85,87- 106]. Table 3 shows correlations between different handheld meters and extraction methods in the most important crops. Several of those correlations, such as for Poaceae and Solanaceae families, needs power or exponential functions due to the non-linear influence of leaf structures. Comparing chlorophyll content among species may be inaccurate due to heterogeneity, although high correlations were found for some grasses, legumes, and green leafy vegetables [95,98,99,105].
In general, a handheld meter can be used in controlled environments as well as in field conditions, they measure a small area per sample, but the measurements are quick, do not require high training to process the data, and can be repeated in several leaves of the same plant (such as different parts of the canopy), in different plants, or even on the same leaf area to assess temporal dynamics. Overall, this method is very useful to compare cultivars of the same species, in similar conditions, in analogous leaves, and the same part of the leaf (excluding veins). This approach has been used to examine different developmental stages and different portions of the canopy for rice and soybean [87,89,90]. Besides, handheld measurements might be used as a proxy for photosynthesis rate, which is effective for primary gross productivity in forest communities [107]. In the latter case, the same precautions, such as sampling the same leaf and excluding veins, are required. While these considerations may imply a higher initial time investment, handheld meters require an intermediate amount of time per sample.
Finally, remote sensors quantify chlorophylls based on the changes in spectral reflectance. Overall, these methods detect the wavelength range between ~400 to 2500 nm, varying in spectra resolution (the highest current resolution is ~0.5 nm). Chlorophyll peaks can be analyzed in combination with other regions to decipher reflectance affected by other factors mentioned above, such as leaf structures. There are four main remote sensing techniques, classified according to the operating spectral regions: optical, thermal, radar, and Light Detection and Ranging (LIDAR) (reviewed by [77,108]). Optical remote sensing is the most wellestablished approach for vegetation and chlorophyll mapping [94]. In general, spectral reflectance measurements can be made in controlled environments as well as in field conditions, across a broad range of spatial scales from sub-leaf to the whole canopy in the field. These kinds of measurements are quick, can be repeated on the same sampling area to assess temporal dynamics, and substantially reduce the time per sample, but require more training to process the data. The sensors can be carried on different instruments, from airborne (e.g., satellites, planes, drones) to portable spectroradiometers, which makes them very versatile and allows for high-scale data acquisition, but also can add errors that need to be considered and corrected. The reflectance spectrum is influenced not only by the leaf but also by variations in soil background reflectance, light scattering by surrounding objects, 3-D canopy and plant architecture, atmospheric conditions (such as clouds and dust suspension), as well as by the angles of the sensor and the incident sunlight, which varies with the hour and the season of the measurement [109,110].
Spectral data analysis is usually simplified by the calculation of indices that use only a discrete band combination (often 2 to 4 bands) and aim to cancel the internal reflection effects. Several spectral indices and models have been developed to assess the chlorophyll content. Most of the currently available models were reviewed by [25] and the indices are listed in several reviews such as [111,112]. Those indices may provide by considering one or two bands that vary under the studied condition (e.g. 690-700 nm) and an extra strong quantitation band that is invariable (e.g. nearinfrared, NIR) [39,113,114], in a similar way that the handheld meters do. One of the most frequently used indices to characterize chlorophyll content is the Normalized Difference Vegetation Index (NDVI). NDVI is based on the reflectance contrast between the red and the NIR bands [115]. Although broadband NDVIs can discern broad differences in vegetation conditions [116,117], they are not effective in assessing detailed chlorophyll content of the canopy due to their saturation at a high leaf area index [111]. Most indices vary with the specific environmental and developmental conditions for each species and therefore need to be calibrated and validated with previously established methods for that condition [108,118].
Overall, the use of indices has a cost in accuracy [118]. Some researchers have proposed that using the whole spectrum could increase the validity of the correlations under a wider range of conditions, but this approach would require much more time, training, and informatic resources for the data analysis (which could be worth it for high throughput projects). Nevertheless, remote monitoring of chlorophylls under drought stress by spectral reflectance is achievable and will likely be more accessible in the near future. The potential of remote sensing to capture simultaneous and massive data on the field may be a turning point for the conventional techniques and thus contribute to increasing the genetic gain efficiency [77].
Breeding For Drought Tolerance By Monitoring Chlorophyll Content
After considering the chlorophyll responses to drought, they indicate that plants which maintain relatively higher chlorophyll content than plants under optimal water availability will be more tolerant. We expect that those plants with higher chlorophyll contents will be more likely to have a higher photosynthesis rate and yield. However, that correlation needs to be demonstrated for most of the crops. Additionally, some experimental aspects may affect the final relevance of the screening to monitor chlorophyll content.
First, if the goal is to map QTLs for drought tolerance, it was proposed that the screenings and heritability calculation need to be done under the specific conditions (drought, in this case). For instance, chlorophyll content in barley exhibited high heritability when mapped independently for well-water and water-restricted conditions, but the heritability was reduced when calculated for both conditions altogether [71]. These differences in heritability suggest that different biochemical mechanisms contribute to chlorophyll content under the specific environmental conditions.
Second, most of the secondary characteristics (characteristics correlated with yield, such as chlorophyll content) used for phenotyping in breeding programs may vary with the duration and intensity of water shortage, the phenological stage when the crop is exposed to the stress, among other factors [119], as mentioned before. Therefore, it is critical to define and plan the experimental conditions and the phenological stage of the crop, to carry out an effective screening.
The experimental conditions will limit the kind of drought that the genotype will experience in the screening. The screening can be carried out in the field (in a dry region or under a rainexclusion shelter) or some analogous treatment in greenhouse or growth chambers. Different options have been carefully analyzed for rice by [120], which could be easily extrapolated to other crops. There are some mimicking strategies of water restriction, like treatments with osmotic agents such as sorbitol or polyethylene glycol (PEG) [121,122], in vitro or hydroponic systems. PEG treatment has been proposed to be useful to study the physiological effects of some components of drought stress [121]. However, drought is more complex stress, e. g. the water in the soil has a gradient of availability, and the plant responds accordingly to it. Then, the use of mimicking systems will require validation with a soil-based water deficit treatment. Therefore, each scale has their own advantages. Field experiments highly variable and require the repetition in several years, but it could be done in a similar or the same environment where the crop will be cultivated. In vitro and hydroponic systems are the least variable and allow very fast screenings, however, they are the least comparable to fields. In between, greenhouses and chambers, allow a fast selection with a controlled drought, excluding most of the environmental variation, and ensure to study the tolerance responses to the specific stress. Depending on the crop cycle, those semi-controlled conditions allow to carry out the screening process in several cycles per year which reduce the time to identify tolerant genotypes and reduce the time required for breeding.
Then, to choose the phenological stage when the plant will be exposed to the stress, we can try to mimic the moment when the stress more often happened on the fields for that crop. The screening design needs to be different for one crop exposed to drought during the seedling establishment than other crops that are exposed at the flowering stage. In particular, chlorophylls may be more useful for leafy crops or for grain crops suffering the stress at vegetative or flowering stages, in contrast with crops exposed to post-flowering stress when most of the chlorophylls were already degraded.
Lastly, it is essential to consider the chlorophylls are not homogeneously distributed across the leaf blade or along with the plant shoot [39,87,123]. This variability needs to be monitored, and equivalent samples (same leaf or in a similar developmental stage) can be collected to compare genotypes. In all cases, it is recommended to sample fully expanded (mature) leaves excluding veins, grown under the stress. These considerations are valid for measuring chlorophyll content by using extractive methods or handheld meters, as well as when sampling to validate spectral reflectance approaches.
Conclusion
Monitoring chlorophyll content, together with other traits, is a promising but underexplored strategy to breed for drought tolerance, and adaptable to economic availability depending on the approach. High-throughput technologies to monitor crop traits (such as chlorophyll content) are key to increase productivity and meet the increasing worldwide demand for plant products. However, for low-income countries, which tend to be the most affected by food scarcity and in need of accessible solutions, affordable technologies are critical and some of the methods presented here may be useful to increase yield at a low cost. Lastly, studies that validate different instruments and methods are needed to obtain comparable and robust results for most of the crops.
Acknowledgments
Funding was provided by INTA (Project 2019-PE-E6-I516-001 and 2019-PE-E6-I127-001), Convenio de Asistencia Técnica INTACriadero El Carmen (N°24102), and Fondo para la investigación Científica y Técnica (PICT 2018-1326). JEDI award (Life Science Editors Foundation) to MIM.
REFERENCES
- FAOSTAT. Food and Agriculture Organization for the United Nations.2021.
- Long SP, Marshall-Colon A, Zhu XG. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell. 2015;161(1):56-66.
- Flood PJ, Harbinson J, Aarts MG. Natural genetic variation in plant photosynthesis. Trends palnt sci. 2011;16(6):327-35.
- Long SP, Ainsworth EA, Leakey AD, Nösberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. science. 2006;312(5782):1918-21.
- Tanaka R, Kobayashi K, Masuda T. Tetrapyrrole metabolism in Arabidopsis thaliana. The Arabidopsis book/American Society of Plant Biologists. 2011;9.
- Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol. 2010;61:235-61.
- Hasegawa T, Li T, Yin X, Zhu Y, Boote K, Baker J, et al. Causes of variation among rice models in yield response to CO 2 examined with Free-Air CO 2 Enrichment and growth chamber experiments. Sci Rep. 2017;7(1):1-3.
- Thomey ML, Slattery RA, Köhler IH, Bernacchi CJ, Ort DR. Yield response of field-grown soybean exposed to heat waves under current and elevated [CO2]. Glob Change Biol. 2019;25(12):4352-68.
- Kim W, Iizumi T, Nishimori M. Global patterns of crop production losses associated with droughts from 1983 to 2009. J APPL METEOROL CLIM. 2019;58(6):1233-44.
- Leng G, Hall J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci Total Environ. 2019;654:811-21.
- Anjum SA, Xie XY, Wang LC, Saleem MF, Man C, Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 2011;6(9):2026-32.
- Kapoor D, Bhardwaj S, Landi M, Sharma A, Ramakrishnan M, Sharma A. The impact of drought in plant metabolism: how to exploit tolerance mechanisms to increase crop production. Appl Sci. 2020;10(16):5692.
- Khodadadi S, Chegini MA, Soltani A, Ajam Norouzi H, Sadeghzadeh Hemayati S. Influence of Foliar-Applied Humic Acid and Some Key Growth Regulators on Sugar Beet (Beta vulgaris L.) Under Drought Stress: Antioxidant Defense System, Photosynthetic Characteristics and Sugar Yield. Sugar Tech. 2020;22:765-72.
- Razzaq M, Akram NA, Ashraf M, Naz H, Al-Qurainy F. Interactive effect of drought and nitrogen on growth, some key physiological attributes and oxidative defense system in carrot (Daucus carota L.) plants. Sci. Hortic. 2017;225:373-9.
- Azzeme AM, Abdullah SN, Aziz MA, Wahab PE. Oil palm leaves and roots differ in physiological response, antioxidant enzyme activities and expression of stress-responsive genes upon exposure to drought stress. Acta Physiol Plant. 2016;38(2):52.
- Puangbut D, Jogloy S, Vorasoot N. Association of photosynthetic traits with water use efficiency and SPAD chlorophyll meter reading of Jerusalem artichoke under drought conditions. Agric Water Manag. 2017;188:29-35.
- Naderikharaji R, Pakniyat H, Biabani AR. Effect of drought stress on photosynthetic rate of four rapeseed (Brassica napus) cultivars. Appl Sci. 2008;8(23):4460-3.
- Ashraf M, Mehmood S. Response of four Brassica species to drought stress. Environ Exp Bot. 1990;30(1):93-100.
- Li QM, Liu BB, Wu Y, Zou ZR. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings. J Integr Plant Biol. 2008;50(10):1307-17.
- Mo Y, Wang Y, Yang R, Zheng J, Liu C, Li H, et al. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front Plant Sci. 2016;7:644.
- Arunyanark A, Jogloy S, Vorasoot N, Akkasaeng C, Kesmala T, Patanothai A. Chlorophyll Meter Readings in Peanut Across Different Drought Stress Conditions. Asian J Plant Sci. 2009;8(2):102-10.
- Songsri P, Jogloy S, Vorasoot N, Akkasaeng C, Patanothai A, Holbrook CC. Root distribution of drought-resistant peanut genotypes in response to drought. J Agron Crop Sci. 2008;194(2):92-103.
- López CM, Pineda M, Alamillo JM. Differential Regulation of Drought Responses in Two Phaseolus vulgaris Genotypes. Plants. 2020;9(12):1815.
- Guzzo MC, Costamagna C, Salloum MS, Rotundo JL, Monteoliva MI, Luna CM. Morpho-physiological traits associated with drought responses in soybean. Crop Sci. 2021;61(1):672-88.
- Zhang J, Liu J, Yang C, Du S, Yang W. Photosynthetic performance of soybean plants to water deficit under high and low light intensity. S AFR J BOT. 2016;105:279-87.
- Kashiwagi J, Upadhyaya HD, Krishnamurthy L. Significance and genetic diversity of SPAD chlorophyll meter reading in chickpea germplasm in the semi-arid environments. Journal of Food Legumes. 2010;23(2):99-105.
- Kumar J, Basu PS, Srivastava E, Chaturvedi SK, Nadarajan N, Kumar S. Phenotyping of traits imparting drought tolerance in lentil. Crop Pasture Sci. 2012;63(6):547-54.
- Li RH, Guo PG, Michael B, Stefania G, Salvatore C. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric Sci China. 2006;(10):751-7.
- Hasanuzzaman M, Shabala L, Brodribb TJ, Zhou M, Shabala S. Assessing the suitability of various screening methods as a proxy for drought tolerance in barley. Funct Plant Biol. 2017;44(2):253-66.
- Geravandi M, Farshadfar E, Kahrizi D. Evaluation of some physiological traits as indicators of drought tolerance in bread wheat genotypes. Russ J Plant Physiol. 2011;58(1):69-75.
- Keyvan S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 2010;8(3):1051-60.
- Talebi R. Evaluation of chlorophyll content and canopy temperature as indicators for drought tolerance in durum wheat (Triticum durum Desf.). AJBAS. 2011;5(11):1457-62.
- Kendal E. Relationship between chlorophyll and other features in durum wheat (Triticum turgidum L. var. durum) using SPAD and biplot analyses. 2015; 17(6):1873-1886
- O'Neill PM, Shanahan JF, Schepers JS. Use of chlorophyll fluorescence assessments to differentiate corn hybrid response to variable water conditions. Crop Sci. 2006;46(2):681-7.
- Avramova V, AbdElgawad H, Zhang Z, Fotschki B, Casadevall R, Vergauwen L, et al. Drought induces distinct growth response, protection, and recovery mechanisms in the maize leaf growth zone. Plant Physiol. 2015;169(2):1382-96.
- Talwar HS, Ashok S, Seetharama N. Use of SPAD chlorophyll meter to screen sorghum (Sorghum bicolor) lines for postflowering drought tolerance. Indian J Agric Sci. 2009;79(1):35-9.
- Silva MD, Jifon JL, Da Silva JA, Sharma V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz J Plant Physiol. 2007;19(3):193-201.
- Rolando JL, Ramírez DA, Yactayo W, Monneveux P, Quiroz R. Leaf greenness as a drought tolerance related trait in potato (Solanum tuberosum L.). Environ Exp Bot. 2015;110:27-35.
- Liew OW, Chong PC, Li B, Asundi AK. Signature optical cues: emerging technologies for monitoring plant health. Sensors. 2008;8(5):3205-39.
- Tanaka R, Kobayashi K, Masuda T. Tetrapyrrole metabolism in Arabidopsis thaliana. The Arabidopsis book/American Society of Plant Biologists. 2011;9.
- Kalaji HM, Schansker G, Brestic M, Bussotti F, Calatayud A, Ferroni L, et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth Res. 2017;132(1):13-66.
- Richardson AD, Duigan SP, Berlyn GP. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002;153(1):185-94.
- Gitelson AA, Peng Y, Arkebauer TJ, Schepers J. Relationships between gross primary production, green LAI, and canopy chlorophyll content in maize: Implications for remote sensing of primary production. Remote Sens Environ. 2014;144:65-72.
- Liu G, Yang C, Xu K, Zhang Z, Li D, Wu Z,et al. Development of yield and some photosynthetic characteristics during 82 years of genetic improvement of soybean genotypes in northeast China. Aust J Crop Sci. 2012;6(10):1416-22.
- Ma BL, Morrison MJ, Voldeng HD. Leaf greenness and photosynthetic rates in soybean. Crop Sci. 1995;35(5):1411-4.
- Bai H, Purcell LC. Evaluation of Soybean Greenness from Ground and Aerial Platforms and the Association with Leaf Nitrogen Concentration in Response to Drought. Crop Sci. 2019;59(6):2763-73.
- Fridgen JL, Varco JJ. Dependency of cotton leaf nitrogen, chlorophyll, and reflectance on nitrogen and potassium availability. Agronomy Journal. 2004;96(1):63-9.
- Reeves DW, Mask PL, Wood CW, Delaney DP. Determination of wheat nitrogen status with a hand-held chlorophyll meter: Influence of management practices. J Plant Nutr. 1993;16(5):781-96.
- Rorie RL, Purcell LC, Mozaffari M, Karcher DE, King CA, Marsh MC, et al. Association of “greenness” in corn with yield and leaf nitrogen concentration. Agronomy Journal. 2011;103(2):529-35.
- Bing YU, Wei-Ya XU, Li-Jun LU, Yong-Zhong XI. QTL analysis for flag leaf characteristics and their relationships with yield and yield traits in rice. Acta Genetica Sinica. 2006;33(9):824-32.
- Hak R, Karlova U, Rinderle-Zimmer U, Lichtenthaler HK, Natr L. Chlorophyll a fluorescence signatures of nitrogen deficient barley leaves. Photosynthetica. 1993;28(1):151-159.
- Kaler AS, Abdel-Haleem H, Fritschi FB, Gillman JD, Ray JD, Smith JR, Purcell LC. Genome-wide association mapping of dark green color index using a diverse panel of soybean accessions. Sci Rep. 2020;10(1):5166.
- Wang P, Richter AS, Kleeberg JR, Geimer S, Grimm B. Post-translational coordination of chlorophyll biosynthesis and breakdown by BCMs maintains chlorophyll homeostasis during leaf development. Nat Commun. 2020;11(1):1254.
- Brzezowski P, Richter AS, Grimm B. Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim Biophys Acta. 2015;1847(9): 968-985.
- Thomas H, Howarth CJ. Five ways to stay green. J Exp Bot. 2000;51(1):329-337.
- Wang P, Grimm B. Organization of chlorophyll biosynthesis and insertion of chlorophyll into the chlorophyll-binding proteins in chloroplasts. Photosynth Res. 2015;126(2):189-202.
- Hörtensteiner S, Kräutler B. Chlorophyll breakdown in higher plants. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2011;1807(8):977-988..
- Meguro M, Ito H, Takabayashi A, Tanaka R, Tanaka A. Identification of the 7-hydroxymethyl chlorophyll a reductase of the chlorophyll cycle in Arabidopsis. The Plant Cell. 20111;23(9):3442-3453.
- Hauenstein M, Christ B, Das A, Aubry S, Hörtensteiner S. A role for TIC55 as a hydroxylase of phyllobilins, the products of chlorophyll breakdown during plant senescence. The Plant Cell. 2016;28(10):2510-2527.
- Hörtensteiner S. Chlorophyll degradation during senescence. Annu Rev Plant Biol. 2006;57(1):55-77.
- Pruinská A, Tanner G, Aubry S, Anders I, Moser S, Müller T, et al. Chlorophyll breakdown in senescent Arabidopsis leaves. Characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol. 2005;139(1):52-63.
- Chen GE, Canniffe DP, Barnett SF, Hollingshead S, Brindley AA, Vasilev C, et al. Complete enzyme set for chlorophyll biosynthesis in Escherichia coli. Science Adv. 2018;4(1):eaaq1407.
- Kuai B, Chen J, Hörtensteiner S. The biochemistry and molecular biology of chlorophyll breakdown. Journal of Experimental Botany. 2018; 69(4): 751-767.
- Mach J. Phytol from Degradation of Chlorophyll Feeds Biosynthesis of Tocopherols. The Plant Cell. 2015;27(10): 2676.
- Woodson JD, Joens MS, Sinson AB, Gilkerson J, Salomé PA, Weigel D, et al. Ubiquitin facilitates a quality-control pathway that removes damaged chloroplasts. Science. 2015;350(6259):450-454.
- Lim PO, Kim HJ, Nam HG. Leaf senescence. Annu Rev Plant Biol. 2007;58(1):115-136.
- Parry C, Blonquist Jr JM, Bugbee B. In situ measurement of leaf chlorophyll concentration: analysis of the optical/absolute relationship. Plant, cell & environment. 2014;37(11):2508-2520.
- Muhammad I, Shalmani A, Ali M, Yang QH, Ahmad H, Li FB. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front Plant Sci. 2021;11(1):2310.
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, et al. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci. 2007;104(49):19631-19636.
- Sharma A, Kumar V, Shahzad B, Ramakrishnan M, Sidhu GP, Bali AS, Handa N, Kapoor D, Yadav P, Khanna K, Bakshi P. Photosynthetic response of plants under different abiotic stresses: A review. J Plant Growth Regul. J Plant Growth Regul. 2020;39(1): 509-531.
- Guo P, Baum M, Varshney RK, Graner A, Grando S, Ceccarelli S. QTLs for chlorophyll and chlorophyll fluorescence parameters in barley under post-flowering drought. Euphytica. 2008;163(2):203-124.
- Batra NG, Sharma V, Kumari N. Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J Plant Interact. 2014;9(1):712-721.
- Taylor SH, Ripley BS, Woodward FI, Osborne CP. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant, Cell & Environment. 2011;34(1):65-75.
- Ramírez DA, Yactayo W, Gutiérrez R, Mares V, De Mendiburu F, Posadas A, et al. Chlorophyll concentration in leaves is an indicator of potato tuber yield in water-shortage conditions. Scientia Horticulturae. 2014;168(1):202-209.
- Li RH, Guo PG, Michael B, Stefania G, Salvatore C. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agricultural Sciences in China. 2006;5(10):751-757.
- Batra NG, Sharma V, Kumari N. Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J Plant Interactions. 2014;9(1):712-721.
- Avramova V, AbdElgawad H, Zhang Z, Fotschki B, Casadevall R, Vergauwen L, et al. Drought induces distinct growth response, protection, and recovery mechanisms in the maize leaf growth zone. Plant Physiol. 2015;169(2):1382-1396.
- Taylor SH, Ripley BS, Woodward FI, Osborne CP. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant, Cell & Environment. 2011;34(1):65-75.
- Guzzo MC, Costamagna C, Salloum MS, Rotundo JL, Monteoliva MI, Luna CM. Morpho-physiological traits associated with drought responses in soybean. Crop Science. 2020;61(1): 672-688.
- Ramírez DA, Yactayo W, Gutiérrez R, Mares V, De Mendiburu F, Posadas A, Quiroz R. Chlorophyll concentration in leaves is an indicator of potato tuber yield in water-shortage conditions. Scientia Horticulturae. 2014;168(1):202-209.
- Fang Y, Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci. 2015;72(4):673-689.
- Thomas H, Howarth CJ. Five ways to stay green. Journal of experimental botany. 2000;51(1):329-337.
- Muhammad I, Shalmani A, Ali M, Yang QH, Ahmad H, Li FB. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front Plant Sci. 2021;11(1):2310.
- Sade N, del Mar Rubio-Wilhelmi M, Umnajkitikorn K, Blumwald E. Stress-induced senescence and plant tolerance to abiotic stress. J Exp Bot. 2018;69(4):845-853.
- Bänziger M, Edmeades GO, Lafitte HR. Selection for drought tolerance increases maize yields across a range of nitrogen levels. Crop Science. 1999;39(4):1035-1040.
- Borrell AK, Hammer GL, Henzell RG. Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Science. 2000;40(4):1037-1348.
- Jordan DR, Hunt CH, Cruickshank AW, Borrell AK, Henzell RG. The relationship between the stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments. Crop Science. 2012;52(3):1153-1161.
- Karlen SD, Zhang C, Peck ML, Smith RA, Padmakshan D, Helmich KE, et al. Monolignol ferulate conjugates are naturally incorporated into plant lignins. Science advances. 2016;2(10):e1600393.
- Ramkumar MK, Senthil Kumar S, Gaikwad K, Pandey R, Chinnusamy V, Singh NK, et al. A novel stay-green mutant of rice with delayed leaf senescence and better harvest index confers drought tolerance. Plants. 2019;8(10):375.
- Yuan Z, Cao Q, Zhang K, Ata-Ul-Karim ST, Tian Y, Zhu Y, et al. Optimal leaf positions for SPAD meter measurement in rice. Front Plant Sci. 2016;7(1):719.
- León AP, Viña SZ, Frezza D, Chaves A, Chiesa A. Estimation of Chlorophyll Contents by Correlations between SPAD-502 Meter and Chroma Meter in Butterhead Lettuce. Communications in Soil Science and Plant Analysis. 2007;38(19-20):2877-2885.
- Mendoza-Tafolla RO, Juarez-Lopez P, Ontiveros-Capurata RE, Sandoval-Villa M, Iran AT, Alejo-Santiago G. Estimating nitrogen and chlorophyll status of romaine lettuce using SPAD and at LEAF readings. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2019;47(3):751-756.
- Cassol D, De Silva FS, Falqueto AR, Bacarin MA. An evaluation of non-destructive methods to estimate total chlorophyll content. Photosynthetica. 2008;46(4):634.
- Fritschi FB, Ray JD. Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica. 2007;45(1):92-98.
- Inoue Y, Guérif M, Baret F, Skidmore A, Gitelson A, Schlerf M, et al. Simple and robust methods for remote sensing of canopy chlorophyll content: A comparative analysis of hyperspectral data for different types of vegetation. Plant, Cell & Environment. 2016;39(1):2609-2623.
- Monje OA, Bugbee B. Inherent limitations of nondestructive chlorophyll meters: A comparison of two types of meters. Hort Science. 1992;27(1):69-71.
- Xu W, Rosenow DT, Nguyen HT. Stay green trait in grain sorghum: relationship between visual rating and leaf chlorophyll concentration. Plant Breeding. 2000;119(4):365-367.
- Cartelat A, Cerovic ZG, Goulas Y, Meyer S, Lelarge C, Prioul JL, et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crops Res. 2005;91(1):35-49.
- Cerovic ZG, Masdoumier G, Ghozlen NB, Latouche G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol Plant. 2012;146(3):251-260.
- Markwell J, Osterman JC, Mitchell JL. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth Res. 1995;46(3):467-472.
- Jiang C, Johkan M, Hohjo M, Tsukagoshi S, Maruo T. A correlation analysis on chlorophyll content and SPAD value in tomato leaves. HortResearch. 2017;71:37-42.
- Nauš J, Prokopová J, Rebícek J, Špundová M. SPAD chlorophyll meter reading can be pronouncedly affected by chloroplast movement. Photosynth Res. 2010;105(3):265-271
- Bindi M, Hacour A, Vandermeiren K, Craigon J, Ojanperä K, Selldén G, et al. Chlorophyll concentration of potatoes grown under elevated carbon dioxide and/or ozone concentrations. European Journal of Agronomy. 2002;17(4):319-335.
- Liu ZA, Yang JP, Yang ZC. Using a chlorophyll meter to estimate tea leaf chlorophyll and nitrogen contents. J. Soil Sci. Plant Nutr. 2012;12(2):339-348.
- Wood CW, Reeves DW, Himelrick DG. Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status, and crop yield: a review. Proceedings Agronomy Society of N.Z. 1993; 23:1-9
- Limantara L, Dettling M, Indrawati R, Brotosudarmo TH. Analysis on the chlorophyll content of commercial green leafy vegetables. Procedia Chemistry. 2015;14:225-231.
- Croft H, Chen JM, Luo X, Bartlett P, Chen B, Staebler RM. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob Chang Biol. 2017;23(9):3513-3524.
- Aasen H, Honkavaara E, Lucieer A, Zarco-Tejada PJ. Quantitative remote sensing at ultra-high resolution with UAV spectroscopy: a review of sensor technology, measurement procedures, and data correction workflows. Remote Sens. 2018;10(7):1091
- Myneni RB, Maggion S, Iaquinta J, Privette JL, Gobron N, Pinty B, Kimes DS, Verstraete MM, Williams DL. Optical remote sensing of vegetation: modeling, caveats, and algorithms. Remote sensing of environment. 1995;51(1):169-188.
- Ollinger SV. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011;189(2):375-394.
- Lu S, Lu F, You W, Wang Z, Liu Y, Omasa K. A robust vegetation index for remotely assessing chlorophyll content of dorsiventral leaves across several species in different seasons. Plant Methods. 2018;14(1):1-5.
- Xue J, Su B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017;2017.
- Carter GA, Miller RL. Early detection of plant stress by digital imaging within narrow stress-sensitive wavebands. Remote Sens. Environ. 1994;50(3):295-302.
- El-Hendawy S, Elsayed S, Al-Suhaibani N, Alotaibi M, Tahir MU, Mubushar M, et al. Use of Hyperspectral Reflectance Sensing for Assessing Growth and Chlorophyll Content of Spring Wheat Grown under Simulated Saline Field Conditions. Plants. 2021;10(1):101.
- Tucker CJ. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979;8(2):127-150
- Main R, Cho MA, Mathieu R, O’Kennedy MM, Ramoelo A, Koch S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J Photogramm Remote Sens. 2011;66(6):751-761.
- Mutanga O, Skidmore AK. Hyperspectral band depth analysis for a better estimation of grass biomass (Cenchrus ciliaris) measured under controlled laboratory conditions. Int. J. Appl. Earth Obs. Geoinf. 2004;5(2):87-96.
- Berger K, Atzberger C, Danner M, D’Urso G, Mauser W, Vuolo F, et al. Evaluation of the PROSAIL model capabilities for future hyperspectral model environments: A review study. Remote Sens. 2018;10(1):85
- Lafitte R. Managing water for controlled drought in breeding plots. Breeding rice for drought-prone environments. 2003:23.
- Lafitte R, Blum A, Atlin G. Using secondary traits to help identify drought-tolerant genotypes. Breeding rice for drought-prone environments. 2003:37-48.
- Dubois M, Inzé D. Plant growth under suboptimal water conditions: early responses and methods to study them. J. Exp. Bot. 2020;71(5):1706-1722
- Munns R, James RA, Sirault XR, Furbank RT, Jones HG. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J. Exp. Bot. 2010;61(13):3499-3507.
- Gabriel JL, Quemada M, Alonso-Ayuso M, Lizaso JI, Martín-Lammerding D. Predicting N status in maize with clip sensors: Choosing sensor, leaf sampling point, and timing. Sensors (Basel). 2019;19(18):3881.
Citation: Monteoliva MI, Guzzo MC, Posada GA (2021) Breeding for Drought Tolerance by Monitoring Chlorophyll Content. Gene Technol. 10:165.
Copyright: © 2021 Monteoliva MI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.