Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Commentary Article - (2021) Volume 13, Issue 6
Bioprocessing 4.0 and the Benefits of Introducing AI to Biopharmaceutical Manufacturing: A Commentary
Dorota Owczarek*Received: 09-Dec-2021 Published: 30-Dec-2021
Description
Bioprocesses are the heart of biotechnology and pharmaceuticals, and there is significant interest in developing novel technologies to improve these processes. Bioprocessing players from the pharmaceutical industry must innovate to remain competitive, but how can they safely and quickly take advantage of new technologies? The biopharmaceutical industry has responded by embracing the future with Bioprocessing 4.0 solutions that incorporate cognitive automation. Intelligent automation involves a range of manufacturing technology improvements, including artificial intelligence, machine learning, deep learning, big data analytics, and the Internet of Things (IoT).
The biopharmaceutical industry strives to keep up with the growing demand and price for biotherapeutics by enhancing manufacturing process performance [1]. To assess upstream and downstream processes and move towards continuous bioprocessing, new equipment, sampling procedures, and analyzers have been developed. On the other hand, these analytical tools produce big, complicated datasets with multivariate interactions. The inherently intricate nature of these data makes extracting valuable and relevant information challenging. Complex multivariate tools are necessary to maintain continuous bioprocesses within a known operational state over the whole process stream.
Smart AI use could benefit bioprocessing
The introduction of AI solutions into bioprocessing has made it easier than ever before to analyze multivariate data from various sources, allowing organizations to utilize this information to improve their processes and product quality. Bioprocessing experts have been using AI techniques such as pattern recognition, ML algorithms (e.g., multivariate data analysis), or more complex deep neural networks along with other signal processing methods for real-time process monitoring to create an understanding of critical process parameters [2]. Novel techniques include predictive and prescriptive modeling based on deep learning techniques that aid manufacturing processes improving efficiency and product quality, and reduce production waste.
Deep learning algorithms used for manufacturing process monitoring aim to generate insights into how process parameters affect final product quality, predict deteriorations, and act on them proactively (Figure 1).
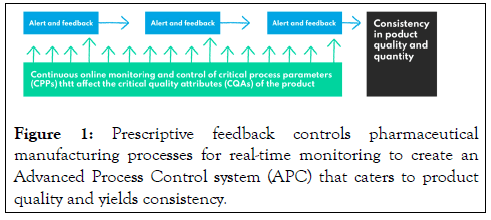
Figure 1: Prescriptive feedback controls pharmaceutical manufacturing processes for real-time monitoring to create an Advanced Process Control system (APC) that caters to product quality and yields consistency.
Any unusual process action may be detected in real-time and recovered via feedback or feed forward control pathways to guarantee quality at the end product. Artificial intelligence algorithms are trained on historical data from a plant's environment sensors to predict future values based on past performance patterns. For that purpose, the time-series data mining technique is usually applied to analyze historical records for pattern recognition and anomaly detection. Process monitoring and APC enable better understanding and consistency in product quality and quantity.
Advanced data-driven modeling based on artificial intelligence solutions can also be used to monitor and predict the entire biopharmaceutical manufacturing process comprehensively, followed by Advanced Process Control. This is especially important in continuous manufacturing, and the growing interest drives the development of AI solutions. Machine learning models trained on data sets from bioreactors can be used to automate the control of large-scale reactors and provide innovative pharmaceutical development processes based on continuous manufacturing [3].
Integrating all available data and process control modeling is now possible by applying custom-made models for specific product lines or using available ready-to-use solutions. The benefit of applying advanced AI models rather than standard MVDA is the possibility of leveraging massive amounts of data into improved process understanding and moving towards a prescriptive, innovative pharmaceutical development process. Several AI solutions can do all of this, and they are designed to adapt or learn automatically to new data sets and create new causal links between process parameters and process variables, in which the underlying algorithms will be utilized.
Anomaly detection and predictive analytics are already in use by pharma companies. To improve the current production processes, a biopharmaceutical active pharmaceutical ingredients producer approached Nexocode to implement AI models to analyze spectral data and reactors' operating parameters. Our challenge was to build a model that analyzes real-time data streams from the chemometric techniques and identifies potential outliers that may lead to quality deterioration based on historical data. The target of creating data pipelines and the model was translating the optical or spectral data into meaningful information that helps maintain an innovative pharmaceutical development process. With high confidence, the model can detect early that the batch will not meet the required quality levels and stop the production process [4]. The benefits are improved efficiency, predictability, and quality assurance of manufacturing operations and yields.
Conclusion
The challenges of implementing custom AI solutions to bioprocessing include the cost of acquisition and modifications to existing infrastructure, long-term maintenance, and training staff. However, the future trends of AI in industrial applications for biopharma look very promising and have the potential to bring superior benefits. The future of AI solutions in bioprocessing is to provide autonomous control and automated feedback and correction mechanisms applied to the manufacturing process.
REFERENCES
- Wasalathanthria DP, Ding J, Li ZJ. Real time process monitoring in biologics development. Am Pharm Rev.2020.
- Richelle A, David B, Demaegd D, Dewerchin M, Kinet R, Morreale A, et al. Towards a widespread adoption of metabolic modeling tools in biopharmaceutical industry: A process systems biology engineering perspective. NPJ Syst Biol Appl. 2020.
- Richelle A, Stosch MV. From big data to precise understanding: The quest for meaningful information. Bio Process int. 2020.
- Lohmann LJ, Strube J. Accelerating biologics manufacturing by modeling: Process integration of precipitation in mab downstream processing. Processes. 2020;8(1):58.
Citation: Owczarek D. (2021) Bioprocessing 4.0 and the Benefits of Introducing AI to Biopharmaceutical Manufacturing: A Commentary. J Bioequiv Availab. 13: 443.
Copyright: © 2021 Owczarek D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

