Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- SWB online catalog
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Article - (2022) Volume 11, Issue 2
Biological Therapies: Induced Autoimmune Adverse Manifestations
Alexandros A. Drosos*, Eleftherios Pelechas and Paraskevi V. VoulgariReceived: 11-Feb-2022, Manuscript No. PDS-22-15711; Editor assigned: 14-Feb-2022, Pre QC No. PDS-22-15711 (PQ); Reviewed: 28-Feb-2022, QC No. PDS-22-15711; Revised: 07-Mar-2022, Manuscript No. PDS-22-15711 (R); Published: 14-Mar-2022, DOI: 10.35248/2167-1052.22.11.264
Abstract
Biological therapies are increasingly used for the treatment of inflammatory arthritides and certain autoimmune rheumatic diseases. Biological agents target specific components of the immune system such as cytokines and their receptors, as well as T and B cells. Thus, the function of an intact immune system is altered and many autoimmune adverse manifestations and paradoxical reactions can take place. In this narrative mini review, we highlight the most common adverse manifestations that may develop during the use of biological therapies while treating rheumatic diseases. Under these circumstances, close follow-up and monitoring and a thorough clinical evaluation are mandatory for early recognition and treatment of these disorders.
Keywords
Biological therapies; Autoimmune adverse manifestations; Paradoxical inflammation; Side effects
Introduction
In the last two decades biological therapies are increasingly used for a number of Autoimmune Rheumatic Diseases (ARD), which achieved a great improvement in treating these disorders [1,2]. Among them are the biologic (b) Disease-Modifying Anti- Rheumatic Drugs (DMARDs), Targeting Tumor Necrosis Factor alpha (TNFα), Interleukin (IL)-1, IL-6, IL-17, IL-12/23 as well as T and B cells [3,4]. The use of bDMARDs has revolutionized the treatment of Rheumatoid Arthritis (RA), Spondylo Arthropathies (SpA), psoriasis, Psoriatic Arthritis (PsA), Inflammatory Bowel Disease (IBD), as well as Systemic Lupus erythematosus (SLE) and vasculitis [3-7]. On the other hand, targeting cytokines or T and B cells, which have a key role for the milieu of the immune system, adverse reactions may occur [8]. When using bDMARDs, predisposition to infections such as viral, bacterial and opportunisticis a matter of concern [9]. Furthermore, many autoimmune adverse manifestations and diseases have emerged during the use of bDMARDs ranging from the discovery of an isolated autoantibody, mostly Antinuclear Antibodies (ANA), to organ specific (e.g. uveitis), or systemic ARDs such as SLE and vasculitis [10,11]. In this short narrative review, we will discuss the most common autoimmune adverse manifestations occurring during the treatment with bDMARDs, especially TNFα inhibitors (TNFαi), but also with other biological therapies.
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All presented material is published after written consent of the patients, although sensitive data and personal details are not included in the publication.
Biological Agents Used to Treat Rheumatic Diseases
The most commonly used agents are TNFαi which comprise the monoclonal Antibodies (mAbs), Adalimumab (ADA), Golimumab (GOL), Infliximab (INF), the soluble TNFα receptor igG Fc fusion protein Etanercept (ETN), and the PEGylated antibody fragment Certolizumab (CTZ). Anakinra is an IL-1 receptor antagonist, while canakinumab is a mAb against IL-1. Tocilizumab (TCZ) and sarilumab (SAR) are IL-6 receptor antagonists. Ustekinumab is a mAb targeting IL-12/23, while secukinumab and ixekizumab are mAbs against IL-17A. Abataceptis acytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) igG fusion protein interfering with T-cells, while rituximab is a mAb targeting CD20 molecule of B-cells and belimumab targets the B-cell activator factor (BAFF) [3-7,12,13]. Furthermore, several bio-similars mostly ADA and ETN, as well as rituximab biosimilar have been developed and approved for the treatment of ARD [14,15] (Table 1).
| TNFα inhibitors | IL-1 inhibitors | IL-6 inhibitors | IL-12/23 inhibitors | IL-17 inhibitors | T-cell inhibitors | B-cell inhibitors |
|---|---|---|---|---|---|---|
| Adalimumab | Anakinra | Tocilizumab | Ustekinumab | Secukinumab | Abatacept | Rituximab |
| Golimumab | ||||||
| Infliximab | Canakinumab | Sarilumab | Ixekizumab | Belimumab | ||
| Etanercept | ||||||
| Certolizumab |
Table 1: Biologic drugs used in autoimmune rheumatic diseases.
Autoimmune Adverse Manifestations and Disease Development
Autoimmune adverse manifestations and the development of diseases like SLE, Antiphospholipid Syndrome (APS), vasculitis and others are often reported to occur during the treatment with TNFαi [11,12,16,17].
The clinical manifestations may appear several weeks or months after the initiation of TNFα blocker sand are the result of a reaction characterized by signs and symptoms as well as serological demonstratoin of et hdisease.
Lupus is a disease that can be developed not uncommonly after treatment with these agents. Its diagnosis requires the identification of a temporal association between TNFαi administration and the development of symptoms in an individual without pre-existing lupus [11,12].
The clinical manifestations of lupus are expressed mostly with erythematous skin lesions affecting the face in a butterfly distribution, the arms, the upper part of the thorax but also other parts of the body, in a photosensitivity distribution (Figures 1 and 2) [18-24].
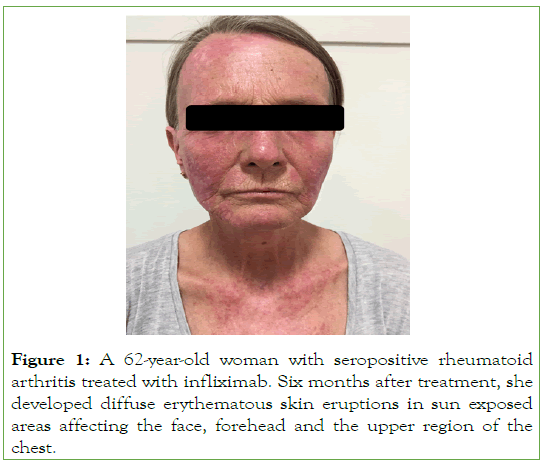
Figure 1: A 62-year-old woman with seropositive rheumatoid arthritis treated with infliximab. Six months after treatment, she developed diffuse erythematous skin eruptions in sun exposed areas affecting the face, forehead and the upper region of the chest.
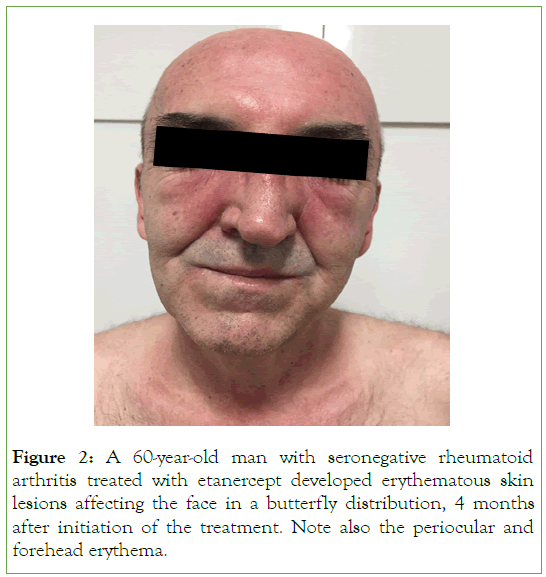
Figure 2: A 60-year-old man with seronegative rheumatoid arthritis treated with etanercept developed erythematous skin lesions affecting the face in a butterfly distribution, 4 months after initiation of the treatment. Note also the periocular and forehead erythema.
In addition, annular or psoriasis form rashes as well as discoid lesions may develop (Figure 3) [25]. Other clinical manifestations include arthralgia as, synovitis, myalgia as, constitutional symptoms, while pleurisy, kidney, or Central Nervous System (CNS) diseases are not uncommon.
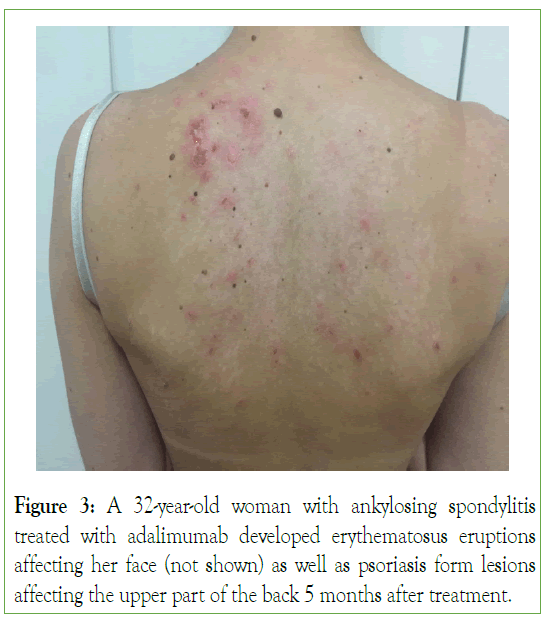
Figure 3: A 32-year-old woman with ankylosing spondylitis treated with adalimumab developed erythematosus eruptions affecting her face (not shown) as well as psoriasis form lesions affecting the upper part of the back 5 months after treatment.
The occurrence of lupus after TNFαi treatment varies between studies ranging from 0.1%-1%, while the presence of ANA ranges from 39%-100% and double stranded (ds) DNA from 10%-50% [11,12,26-30].
The occurrence of APS is approximately 1%, while the presence of anti-Cardiolipin (aCl) antibodies between 7%-12% [16,17]. As far as it concerns the treatment, in patients with mild manifestations of lupus, discontinuation of the offended TNF-α blocker may be a sufficient measure. In some cases, small doses of prednisone with or without topical use of calcineurin inhibitors may be useful. In patients who develop systemic disease with kidney or CNS manifestations, high doses of prednisone along with immunosuppressive drugs are required [11,12].
The involved pathogenetic mechanism for the development of lupus after TNFαi is not well understood. One hypothesis implies that TNFαi, by blocking the TNFα cell membrane, can induce cell apoptosis releasing antigenic material which acts as a foreign antigen, leading to the generation of autoantibodies and the development of lupus. Another hypothesis is that TNFαi can interfere with Th1/Th2 response, leading to the production of Interferon (IFN) α, which is involved in the pathogenesis of lupus [31-33].
Paradoxical Inflammation
Paradoxical inflammation is an intriguing adverse event of biological therapies. It mostly occurs during treatment with TNFαi. It is presented as the same type of clinical manifestations for which the agents are effectively used for. Patients with RA, psoriasis, SpA and IBD may be affected [34-36]. Thus, paradoxical inflammation can be manifested as psoriasis, colitis, arthritis, uveitis and several other dermatological clinical features [34-36]. The most common is the development of psoriasis form skin lesions affecting mainly the palmoplantar regions, scalp, hands butother skin areas may also be affected (Figures 4 and 5) [37,38]. The occurrence of psoriatic skin lesions in RA patients treated with TNF-α blockers range between 0.6%-5.3%, in SpA 4%, while in IBD between 1.6%-10% [10]. The pathogenesis of paradoxical inflammation following TNFαi has not been fully elucidated. It is postulated that TNFαi may cause a cytokine shift of TNFα and IFNα. It is well demonstrated that plasmacytoid cells produce high amounts of IFNα, which accumulate in the skin, and it is responsible for psoriasis development. On the contrary, TNFα prevents the generation of plasmacytoid dendritic cells and down-regulate the production of IFNα. Thus, by blocking TNFα using TNFαi, the production of IFNα is increased by plasmacytoid cells with the development of psoriasis [10]. TNFαi bio-similars have been reported to induce the same adverse event like the bio-originators (Figure 4). Indeed, SB4 an ETN bio-similars, can induce psoriasis affecting the palms of the hands in a patient suffering from RA [39]. Rituximab is used to treat RA and SLE, among other diseases. It has been reported from case reports, the development of psoriasis form skin lesions during rituximab therapy [40].
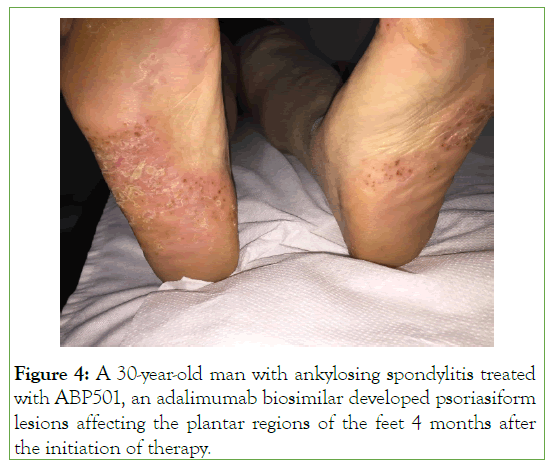
Figure 4: A 30-year-old man with ankylosing spondylitis treated with ABP501, an adalimumab biosimilar developed psoriasiform lesions affecting the plantar regions of the feet 4 months after the initiation of therapy.
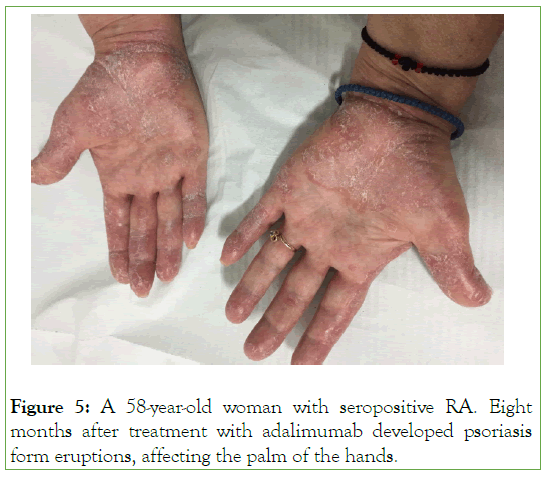
Figure 5: A 58-year-old woman with seropositive RA. Eight months after treatment with adalimumab developed psoriasis form eruptions, affecting the palm of the hands.
Another paradoxical manifestation due to TNFαi use is the development of Granuloma Annulare (GA). Several studies describe GA during ADA, ETN and INF treatment in patients suffering from RA, SpA, and psoriasis [41,42]. GA has been described also in patients with RA receiving secukinumab and TCZ [43,44]. In addition, erythema multi-forme, skin vasculitis, folliculitis, vitiligo, and alopecia have also been described among others [35]. Regarding the treatment, discontinuation of the offended drug may be sufficient in mild cases. However, in some cases small doses of prednisone and topical steroids are required [10].
Neurological Adverse Manifestations
TNFαi may lead to the development of Neurological Diseases (ND) affecting the CNS as well as the peripheral nervous system. Indeed, Demyelinating Diseases (DD), Multiple Sclerosis (MS)- like lesions, myelitis, optic neuritis, as well as mono-neuritis multiplex, sensorimotor polyneuropathy, Myasthenia Gravis (MG) and others have been described in case reports, case series, retrospective and prospective studies [45-50]. The pathophysiological mechanism of this phenomenon is not clear. A hypothesis suggests that TNFαi may increase autoreactive Tcells in the periphery which invade CNS causing MS-like lesions. Another hypothesis implies that TNFαi downregulate IL-10 and upregulate IL-12 and INFγ responsible for this disorder. Finally, TNFαi may cause downregulation of TNF receptor 2 (TNFR2), responsible for oligodendrocytes proliferation and damage repair [10]. Furthermore, the use of rituximab has been reported to develop Progressive Multi-focal Leukoencephalopathy (PML), which is a reactivation of John Cunningham Virus (JCV). In these disorders discontinuation of the responsible agent is mandatory [51-53].
Other Autoimmune Adverse Manifestations
In addition to the afore mentioned paradoxical autoimmune disorders, inflammatory myopathy, sarcoidosis, interstitial lung disease, and uveitis have also been described in case reports, case series and retrospective studies [54-56].
Conclusion
Nowadays, bDMARDs, especially TNFαi have revolutionized the treatment of inflammatory arthritides and certain ARDs, by demonstrating good efficacy and an acceptable toxicity profile. However, their use may interfere with the immune system functions, thus many autoimmune adverse manifestations, paradoxical reactions and diseases may develop. Between them SLE-like manifestations, psoriasis form reactions, granuloma annulare, MS-like disorders, MG and many others have been described. Thus, physicians using bDMARDs should have their patients in a close follow-up and monitoring, along with a minute and careful examination in order to recognize early these adverse manifestations and to treat them appropriately.
Acknowledgements
The authors thank Ms. Chrysa Arvaniti for her excellent secretarial assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
All authors have made substantial contribution to the current manuscript and have approved the final version fulfilling all the four criteria requested by the ICNJE. EP: drafting, PVV revision, AAD: conception of the work, revision.
Conflict of Interest
All authors declare no conflict of interest.
REFERENCES
- Burmester GR, Feist E, Dörner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(2):77-88.
[CrossRef], [GoogleScholar], [PubMed]
- Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: An update. BMC Med. 2013;11:88.
[CrossRef], [GoogleScholar], [PubMed]
- Sfikakis PP, Bournia VK, Sidiropoulos P, Boumpas DT, Drosos AA, Kitas GD, et al. Biologic treatment for rheumatic disease: real-world big data analysis from the Greek country-wide prescription database. Clin Exp Rheumatol. 2017;35(4):579-585.
[GoogleScholar], [PubMed]
- Flouri I, Markatseli TE, Voulgari PV, Boki KA, Papadopoulos I, Settas L, et al. Comparative effectiveness and survival of infliximab, adalimumab, and etanercept for rheumatoid arthritis patients in the Hellenic Registry of Biologics: low rates of remission and 5-year drug survival. Semin Arthritis Rheum. 2014;43(4):447-457.
[CrossRef], [GoogleScholar], [PubMed]
- Voulgari PV, Drosos AA. Adalimumab for rheumatoid arthritis. Expert Opin Biol Ther. 2006;6(12)1349-1360.
[CrossRef], [GoogleScholar], [PubMed]
- Papagoras Ch, Voulgari PV, Drosos AA. Golimumab, the newest TNF-α blocker, comes of age. Clin Exp Rheumatol. 2015;33(4):570.
[GoogleScholar], [PubMed]
- Markatseli TE, Papagoras C, Nikoli A, Voulgari PV, Drosos AA. Certolizumab for rheumatoid arthritis. Clin Exp Rheumatol. 2014;32(3):415-423.
[GoogleScholar], [PubMed]
- Her M, Kavanaugh A. Alterations in immune function with biologic therapies for autoimmune disease. J Allergy ClinImmunol 2016, 137(1):19-27.
[CrossRef], [GoogleScholar], [PubMed]
- Boyman O, Comte D, Spertini F. Adverse reactions to biologic agents and their medical management. Nat Rev Rheumatol. 2014;10(10):612-627.
[CrossRef], [GoogleScholar], [PubMed]
- Drosos AA, Pelechas E, Kaltsonoudis E, Markatseli TE, Voulgari PV. Biologic Therapies and Autoimmune Phenomena. Mediterr J Rheumatol. 2021;32(2):96-103.
[CrossRef], [GoogleScholar], [PubMed]
- Skalkou A, Pelechas E, Voulgari PV, Drosos AA.TNF-Induced Lupus: A Case-Based Review. Curr Rheumatol Rev. 2021;18(1):72-82.
[CrossRef], [GoogleScholar], [PubMed]
- Markatseli TE, Theodoridou A, Zakalka M, Koukli E, Triantafyllidou E, Tsalavos S, et al. Persistence and adherence during the first six months of tocilizumab treatment among rheumatoid arthritis patients in routine clinical practice in Greece: Results from the single arm REMISSION II Study (NCT01649817). Mediterr J Rheumatol. 2019;30(3):177-185.
[CrossRef], [GoogleScholar], [PubMed]
- Pelechas E, Voulgari PV, Drosos AA. Clinical evaluation of the safety, efficacy and tolerability of sarilumab in the treatment of moderate to severe rheumatoid arthritis. Ther Clin Risk Manag. 2019;15:1073-1079.
[CrossRef], [GoogleScholar], [PubMed]
- Pelechas E, Voulgari PV, Drosos AA. ABP 501 for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2018;18(3):317-322.
[CrossRef], [GoogleScholar], [PubMed]
- Pelechas E, Drosos AA. Etanercept biosimilar SB-4. Expert Opin Biol Ther. 2019;19(3):173-179.
[CrossRef], [GoogleScholar], [PubMed]
- Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007, 86(4):242-251.
[CrossRef], [GoogleScholar], [PubMed]
- Ramos-Casals M, Diaz-Lagares C, Cuadrado MJ, Khamashta MA. BIOGEAS Study Group. Autoimmune diseases induced by biological agents: a double-edged sword? Autoimmun Rev. 2010;9(3):188-193.
[CrossRef], [GoogleScholar], [PubMed]
- Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatol (Oxford). 2009;48(7):716-720.
[CrossRef], [GoogleScholar], [PubMed]
- Cairns AP, Duncan MK, Hinder AE, Taggart AJ. New onset systemic lupus erythematosus in a patient receiving etanercept for rheumatoid arthritis. Ann Rheum Dis. 2002;61(11):1031-1032.
[CrossRef], [GoogleScholar], [PubMed]
- Shakoor N, Michalska M, Harris CA, Block JA. Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet. 2002;359(9306):579-580.
[CrossRef], [GoogleScholar], [PubMed]
- Choi SJ, Ahn SM, Oh JS, et al. Anti-tumor necrosis factor-induced lupus in patients with inflammatory bowel disease: a hospital-based cohort study from Korea. Therap Adv Gastroenterol. 2021;14:1756284821997794.
[CrossRef], [GoogleScholar], [PubMed]
- Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci. 2007;1108:166-182.
[CrossRef], [GoogleScholar], [PubMed]
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21(1):235.
[CrossRef], [GoogleScholar], [PubMed]
- Chang C, Gershwin ME. Drug-induced lupus erythematosus: incidence, management and prevention. Drug Saf. 2011;34(5):357-374.
[CrossRef], [GoogleScholar], [PubMed]
- Bleumink GS, ter Borg EJ, Ramselaar CG, Stricker BH. Etanercept-induced subacute cutaneous lupus erythematosus. Rheumatol (Oxford). 2001;40(11):1317-1319.
[CrossRef], [GoogleScholar], [PubMed]
- Vaz JL, Andrade CA, Pereira AC, Martins MdeF, Levy RA. Systematic review of infliximab-induced autoantibodies and systemic lupus erythematosus. Rev Bras Reumatol. 2013;53(4):358-364.
[CrossRef], [GoogleScholar], [PubMed]
- Beigel F, Schnitzler F, Paul Laubender R, Pfennig S, Weidinger M, Göke B, et al. Formation of antinuclear and double-strand DNA antibodies and frequency of lupus-like syndrome in anti-TNF-α antibody-treated patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):91-98.
[CrossRef], [GoogleScholar], [PubMed]
- Eriksson C, Engstrand S, Sundqvist KG, Rantapää-Dahlqvist S. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha. Ann Rheum Dis. 2005;64(3):403-407.
[CrossRef], [GoogleScholar], [PubMed]
- Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials. Arthritis Rheum. 2000;43(11):2383-2390.
[CrossRef], [GoogleScholar], [PubMed]
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alpha agents. Semin Arthritis Rheum. 2008;37(6):381-387.
[CrossRef], [GoogleScholar], [PubMed]
- D’Auria F, Rovere-Querini P, Giazzon M, Ajello P, Baldissera E, Manfredi AA, et al. Accumulation of plasma nucleosomes upon treatment with anti-tumour necrosis factor-alpha antibodies. J Intern Med. 2004;255(3):409-418.
[CrossRef], [GoogleScholar], [PubMed]
- Wahren-Herlenius M, Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382(9894):819-831.
[CrossRef], [GoogleScholar], [PubMed]
- Chang C, Gershwin ME. Drugs and autoimmunity: A contemporary review and mechanistic approach. J Autoimmun. 2010;34(3):J266-J275.
[CrossRef], [GoogleScholar], [PubMed]
- Brion PH, Mittal-Henkle A, Kalunian KC. Autoimmune skin rashes associated with etanercept for rheumatoid arthritis. Ann Intern Med. 1999;131(8):634.
[CrossRef], [GoogleScholar], [PubMed]
- Exarchou SA, Voulgari PV, Markatseli TE, Zioga A, Drosos AA. Immune-mediated skin lesions in patients treated with anti-tumour necrosis factor alpha inhibitors. Scand J Rhematol. 2009;38(5):328-331.
[CrossRef], [GoogleScholar], [PubMed]
- Fiorino G, Danese S, Pariente B, Allez M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-α agents. Autoimmun Rev. 2014;13(1):15-19.
[CrossRef], [GoogleScholar], [PubMed]
- Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52(8):2513-2518.
[CrossRef], [GoogleScholar], [PubMed]
- De Gannes GC, Ghoreishi M, Pope J, et al. Psoriasis and pustular dermatitis triggered by TNF-{alpha} inhibitors in patients with rheumatologic conditions. Arch Dermatol. 2007;143(2):223-231.
[CrossRef], [GoogleScholar], [PubMed]
- Pelechas E, Papoudou-Bai A, Voulgari PV, Drosos AA. Cutaneous autoimmune phenomena of the anti-TNFα bio similars: Case based Review. Curr Rheumatol Rev. 2021;17(2):267-270.
[CrossRef], [GoogleScholar], [PubMed]
- Markatseli TE, Kaltsonoudis ES, Voulgari PV, Zioga A, Drosos AA. Induction of psoriatic skin lesions in a patient with rheumatoid arthritis treated with rituximab. Clin Exp Rheumatol. 2009;27(6):996-998.
[GoogleScholar], [PubMed]
- Devos SA, van Den Bossche N, de Vos M, Naeyaert JM. Adverse skin reactions to anti-TNF-alpha monoclonal antibody therapy. Dermatol. 2003;206(4):388-390.
[CrossRef], [GoogleScholar], [PubMed]
- Voulgari PV, Markatseli TE, Exarchou SA, Zioga A, Drosos AA. Granuloma annulare induced by anti-tumour necrosis factor therapy. Ann Rheum Dis. 2008;67(4):567-570.
[CrossRef], [GoogleScholar], [PubMed]
- Clark ML, Tobin CA, Sutton A, Missall TA. Granuloma annulare in the setting of secukinumab. Case Rep Dermatol Med. 2018;2018:5175319.
[CrossRef], [GoogleScholar], [PubMed]
- Pelechas E, Papoudou-Bai A, Voulgari PV, Drosos AA. Granuloma annulare development in a patient with rheumatoid arthritis treated with tocilizumab: case-based review. Rheumatol Int. 2019;39(2):353-357.
[CrossRef], [GoogleScholar], [PubMed]
- Andreadou E, Kemanetzoglou E, Brokalaki Ch, Evangelopoulos ME, Kilidireas C, Rombos A, et al. Demyelinating disease following anti-TNFα treatment: a causal or coincidental association? Report of four cases and review of the literature. Case Rep Neurol Med. 2013;2013:671935.
[CrossRef], [GoogleScholar], [PubMed]
- Faillace C, de Almeida JRM, de Carvalho JF. Optic neuritis after infliximab therapy. Rheumatol Int. 2013;33:1101-1103.
[CrossRef], [GoogleScholar], [PubMed]
- Kaltsonoudis E, Zikou AK, Voulgari PV, Konitsiotis S, Argyropoulou MI, Drosos AA. Neurological adverse events in patients receiving anti-TNF therapy: aprospective imaging and electrophysiological study. Arthritis Res Ther. 2014;16(3):R125.
[CrossRef], [GoogleScholar], [PubMed]
- Kaltsonoudis E, Pelechas E, Voulgari PV, Drosos AA. Neuro-inflammatory events after anti-TNFα therapy. Ann Rheum Dis. 2020;annrheumdis-2020-217723.
[CrossRef], [GoogleScholar], [PubMed]
- Kaltsonoudis E, Voulgari PV, Konitsiotis S, Drosos AA. Demyelination and other neurological adverse events after anti-TNFtherapy. Autoimmun Rev. 2014;13(1):54-58.
[CrossRef], [GoogleScholar], [PubMed]
- Pelechas E, Memi T, Markatseli TE, Voulgari PV, Drosos AA. Adalimumab-induced myasthenia gravis: case-based review. Rheumatol Int. 2020;40(11):1891-1894.
[CrossRef], [GoogleScholar], [PubMed]
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies. Arthritis Rheum 2012;64(9):3043-3051.
[CrossRef], [GoogleScholar], [PubMed]
- Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17(12):1776-1780.
[CrossRef], [GoogleScholar], [PubMed]
- Kothary N, Diak IL, Brinker A, Bezabeh S, Avigan M, Pan GD. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65(3):546-551.
[CrossRef], [GoogleScholar], [PubMed]
- Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, et al. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41(2):256-264.
[CrossRef], [GoogleScholar], [PubMed]
- Scrivo R, Spadaro A, Spinelli FR, Valesini G. Uveitis following the use of tumor necrosis factor alpha inhibitors: comment on the article by Lim et al. Arthritis Rheum. 2008;58(5):1555-1556.
[CrossRef], [GoogleScholar], [PubMed]
- Daien CI, Monnier A, Claudepierre P, Constantin A, Eschard JP, Houvenagel E, et al. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatol (Oxford). 2009;48(8):883-886.
[CrossRef], [GoogleScholar], [PubMed]
Citation: Drosos AA, Pelechas E, Voulgari PV (2022) Biological Therapies: Induced Autoimmune Adverse Manifestations. Adv Pharmacoepidemiol Drug Saf. 11:264.
Copyright: © 2022 Drosos AA et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.