Indexed In
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 13, Issue 2
Biological Atrazine Removal from Low-Strength Wastewater by Moving Bed Biofilm Reactor and Upflow Fixed Bed Bioreactor: Performance and Kinetic Analysis
Sara Kamanmalek1*, Ali Dabestani Rahmatabad2 and Seyed Mehdi Borghei32Laboratoire de Biotechnologie de l’Environnement (LBE), INRAE-French National Research Institute for Agriculture, Food and the Environment, Villeneuve-d’Ascq, France
3Department of Chemical and Petroleum Engineering, Sharif University of Technology, Tehran, Iran
Received: 23-Jul-2024, Manuscript No. GJBAHS-24-26570; Editor assigned: 26-Jul-2024, Pre QC No. GJBAHS-24-26570 (PQ); Reviewed: 09-Aug-2024, QC No. GJBAHS-24-26570 ; Revised: 16-Aug-2024, Manuscript No. GJBAHS-24-26570 (R); Published: 23-Aug-2024, DOI: 10.35248/2319- 5584.24.13.226
Abstract
Atrazine is a commonly used herbicide that can pose risks to the environment and human health. Despite the
effectiveness of bioreactors in treating organic compounds, their performance in removing atrazine from lowstrength
wastewater is not yet fully understood. This study investigates the effectiveness of Moving Bed Biofilm
Reactor (MBBR) and upflow Fixed Bed Bioreactor (FBBR) in removing atrazine from low-strength wastewater.
To evaluate the impacts of environmental conditions on atrazine biodegradability, experiments were conducted
at different atrazine concentrations, hydraulic retention times, and nutrient ratios (COD:N:P). All experiments
were conducted at COD of 200 mg/L to evaluate bioreactor effectiveness in removing atrazine from low-strength
wastewater. Additionally, we evaluated the kinetics of atrazine removal by applying the modified Stover-Kincannon
model. The results suggest that both FBBR and MBBR are effective in removing atrazine and COD, with FBBR
showing higher removal efficiency. The average and maximum atrazine removal efficiency was 41.8% and 75.2%
in MBBR, and 48.3% and 81.6% in FBBR, respectively. Higher nitrogen levels decreased atrazine removal, while
higher HRTs and initial atrazine concentrations improved removal efficiency in both bioreactors. The constant
values of modified Stover-Kincannon model for KB and Umax were calculated as 4.15 and 1.49 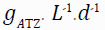 in MBBR,
and 5.73 and 2.30
in MBBR,
and 5.73 and 2.30 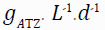 in FBBR. This study contributes to the development of efficient and cost-effective
strategies for wastewater treatment, highlighting the potential of bioreactors as a sustainable technology for atrazine
removal from low-strength wastewater.
in FBBR. This study contributes to the development of efficient and cost-effective
strategies for wastewater treatment, highlighting the potential of bioreactors as a sustainable technology for atrazine
removal from low-strength wastewater.
Keywords
Atrazine; Moving bed biofilm reactor; Upflow fixed bed biofilm reactor; Low-strength wastewater; Stover- Kincannon model
Introduction
Atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) is one of the most widely-used chlorine herbicides in agricultural practices [1,2]. Atrazine has been frequently detected in deep layers of the soil as well as groundwater and surface water due to its excessive use and physicochemical properties such as high solubility and stability in water [3-5]. Atrazine presence can enter aquatic ecosystems through various pathways, including runoff from agricultural lands, leaching into groundwater, and atmospheric deposition [6,7]. Once released into aquatic environments, atrazine can persist for months to years and can accumulate in sediments, aquatic plants, and organisms, resulting in potential environmental and health risks [8-10]. Studies have linked atrazine exposure to a number of health issues in humans, including reproductive problems, birth defects, hormonal imbalances, cancer, ovarian dysfunction, and liver injury [11-13]. Atrazine persistence and toxicity can have negative impacts on ecological health, such as the reduction of biodiversity and the disruption of food webs [14,15]. Atrazine can also affect non-target organisms such as fish, amphibians, and aquatic invertebrates [16,17]. Exposure to atrazine has been linked to developmental abnormalities, reduced reproductive success, and endocrine disruption in these organisms [18,19]. Given these potential impacts, the removal of atrazine from water bodies is crucially important.
Conventional methods for atrazine removal, such as physical and chemical treatments, are expensive and may generate hazardous byproducts [20]. Bioreactors, on the other hand, offer a sustainable and cost-effective alternative by utilizing microorganisms to degrade the contaminant into harmless compounds [21]. Bioreactors have shown potential in removing atrazine from contaminated water sources, making them a potential sustainable solution for atrazine removal [22]. Different types of bioreactors have been used for Atrazine removal from wastewater [23-31]. Biochar-amended denitrifying bioreactor had 90% of Atrazine removal efficiency during 72-h retention time [32]. In an anaerobic membrane bioreactor coupled with forward osmosis, atrazine removal was equal to 93.3% [33]. In lab-scale membrane bioreactors [34], achieved Atrazine removals of less than 36% and 45%, respectively with a hydraulic retention time of 25 hours [29,34]. The higher removal efficiency in the latter study might be due to higher biomass concentration in the Membrane Bioreactor (MBR) (between 9.0 to 19.5 g/L) [29,34], achieved 60.5% of atrazine removal in an anaerobic Moving Bed Biofilm Reactor (MBBR) [34]. In addition, Fixed Bed Biofilm Reactors (FBBRs) have proven to be efficient in removing a range of contaminants from wastewater such as total nitrogen, total phosphorus, Chemical Oxygen Demand (COD), and other organic compounds [35,36]. However, no studies investigated FBBR’s effectiveness in removing atrazine.
The effectiveness of biofilm reactors can be influenced by several key parameters such as temperature, hydraulic retention time, pH, and the choice of microorganisms [37,38]. Therefore, understanding the factors affecting the performance of bioreactors for atrazine removal is essential for the development of efficient and reliable bioreactor systems. One of the important factors impacting atrazine biological removal is the availability of nitrogen [39]. Although nitrogen amendments led to a decrease in atrazine biodegradation [39-41], the impact of nitrogen levels on bioreactor performance in atrazine removal has not yet been assessed. Despite the importance of adapting bioreactors to the treatment of low-strength wastewater [42,43], no study has examined the efficiency of bioreactors in removing atrazine from low-strength wastewater.
Although bioreactors have shown potential for atrazine removal, further research is needed to optimize their design and operation parameters for the effective removal of atrazine and to better understand the impacts of factors affecting their performances. Despite nitrogen amendments decreasing atrazine biodegradation, the impact of nitrogen level on bioreactor performance is unknown. There have been no studies examining the efficiency of bioreactors in removing atrazine from low-strength wastewater or the effectiveness of FBBRs in removing atrazine in general. This study addresses the aforementioned gaps in the bioreactor literature by investigating the effectiveness of MBBR and upflow FBBR in removing atrazine from low-strength wastewater under different operational conditions. To assess the impacts of environmental conditions on atrazine biodegradability, tests were conducted at various initial atrazine concentrations (5, 10, 15, 20, and 30 mg/L), different HRTs (5, 10, and 24 h, and different nutrient ratios (COD to N to P) equal to 100:5:1 and 100:15:1. Moreover, all experiments were conducted with COD of 200 mg/L to evaluate bioreactor effectiveness in removing atrazine from low-strength wastewater. In addition, we have applied the modified Stover-Kincannon model to investigate the kinetics of atrazine elimination by MBBR and FBBR. The results of this research offer valuable knowledge about the potential of bioreactors as a sustainable technology for atrazine removal and contribute to the development of efficient and cost- effective strategies for water remediation.
Materials and Methods
Chemicals and reagents
For this study, all chemicals utilized were of analytical grade and were supplied by Merck. The atrazine standard, which had a purity level of 99.9%, was provided by Sigma-Aldrich. To prepare the stock solution of atrazine, 3 mg of solid standard atrazine was dissolved in 100 mL of methanol, specifically designed for High-Performance Liquid Chromatography (HPLC). Following the filtration process using 0.4 μm filters, the stock solution was transferred and stored in a freezer set at -20°C. The storage container used for this purpose was an amber glass bottle, which helps protect the solution from light exposure. Atrazine solutions used in HPLC calibration were prepared at concentrations between 0.1 mg/L and 100 mg/L by serial dilution. Working solutions were prepared upon necessity by diluting an appropriate volume of stock solution in methanol and kept in a fridge at 4°C.
Bioreactor configuration
To assess atrazine biodegradability and the impact of key parameters on atrazine removal, two lab-scale bioreactors including one MBBR and one up-flow FBBR were used. The configuration and flow diagram of the bioreactors can be found in Figure 1. The MBBR was constructed using cuboidal plexiglass, with total dimensions of 30 × 30 × 45 cm (length × width × height), resulting in a total volume of 40.5 L and a working volume of 27 L. Similarly, the FBBR was also made of cuboidal plexiglass, with total dimensions of 30 cm in length × 30 cm in width × 55 cm in height, resulting in a total volume of 49.5 L and a working volume of 40.5 L. To ensure continuous aeration within the bioreactors, maintaining a dissolved oxygen concentration of approximately 2 mg/L. In addition, each reactor had three air stones (20 cm) placed at the bottom to achieve a fully mixed regime. The Kaldnes biofilm carrier occupied approximately 35% of the working volume in each reactor. This carrier provides a biofilm-specific surface area of 480 m2/m3, with internal dimensions of 41 mm (length) × 21 mm (diameter), and a density equal to 96 g/cm3. Synthetic feed for the reactors was introduced from the top of the MBBR and the bottom of the FBBR, as depicted in Figure 1. Moreover, outlet valves were situated in the middle of the MBBR and at the top of the FBBR. The effluent from each bioreactor was discharged into separate sedimentation tanks with a volume of 30 m3. These tanks were primarily utilized for sludge separation and return, if necessary.
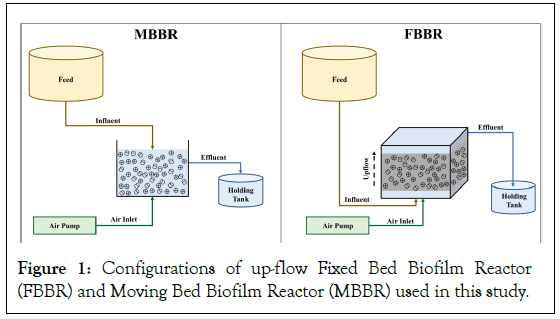
Figure 1: Configurations of up-flow Fixed Bed Biofilm Reactor (FBBR) and Moving Bed Biofilm Reactor (MBBR) used in this study.
Startup and system operation
To set up the system and initiate biofilm growth on the carriers, bioreactors were seeded by an activated sludge collected from Ekbatan municipal wastewater treatment plant (Tehran, Iran). Approximately, half of the working volume of the reactors was occupied by sludge, characterized by COD and Mixed Liquor Suspended Solids (MLSS) equal to 0.5 g/L and 6.05 g/L, respectively. Then, bioreactors were operating in the batch mode for four weeks; while synthetic wastewater was injected every 24 hours consisting of sugar, urea (as nitrogen source), and KH2PO4 (as phosphate source); with a nutrient ratio of COD:N: P=100:5:1, and COD of 1500 mg/L. To ensure microbial activity in this stage and prevent the loss of microorganisms, settled sludge collected in the sedimentation tank was extracted and returned to the bioreactors. At each stage, environmental conditions inside the bioreactors were measured and if necessary, adjusted to optimum values to maintain an adequate microbial population for the treatment of the wastewater. Envy, et al. [44], have reported the maximum atrazine removal at 32°C; therefore, the temperature of the influent was set to 32°C by an electric heater placed in the feed tank. In addition to temperature, several controlled parameters were maintained during the experiment. The pH level was kept within the neutral range by adjusting it using NaOH. The MLSS were maintained at a concentration of 2500 mg/L, and the attached growth had a concentration of 1300 mg/L. At each stage, environmental conditions inside the bioreactors including pH, temperature, MLSS, and dissolved oxygen were measured and if necessary, adjusted to optimum values to maintain an adequate microbial population for the treatment of the wastewater.
After 4 weeks of feeding bioreactors without atrazine, biofilm was formed on the surface of the carriers, and COD removal in bioreactors reached a constant rate. After biofilm formation on carriers, atrazine was added gradually into synthetic feed for acclimation of microorganisms to atrazine presence. The acclimation process started with the injection of synthetic wastewater containing 0.01 mg/L atrazine, COD of 1500 mg/L, and a nutrient ratio of COD:N: P=100:5:1. This procedure was repeated daily (HRT=24 h) until achieving the steady-state performance (i.e. COD removal changes below 3%) and followed by 5 similar consecutive cycles with a gradual increase of atrazine concentration in each cycle (atrazine concentrations: 0.1, 1, 10, and 20 mg/L).
Experimental design
To investigate atrazine removal efficiency in MBBR and FFBR, and to assess the impacts of environmental conditions on atrazine biodegradability, tests were conducted per bioreactor, at five different initial atrazine concentrations: 5, 10, 15, 20, and 30 mg/L; three different HRT:5, 10 and 24 h; and two different COD to N to P ratio equal to 100:5:1 and 100:15:1 (i.e., increase in nitrogen content). Environmental parameters include DO (2 mg/L), pH at the neutral range, attached growth equal to 1300 mg/L, temperature at 32°C, and MLSS of 2500 mg/L. Moreover, all experiments were performed with COD of 200 mg/L in order to evaluate the effectiveness of MBBR and FBBR in removing atrazine from low strength wastewater. Samples were collected after the steady-state performance was achieved which usually took two HRT. Duplicate samples were collected from each bioreactor to ensure Quality Assurance and Quality Control (QA/QC), and COD and atrazine concentrations were reported as the average of duplicate samples. Experimental conditions applied in the study alongside COD concentrations in influent and effluent of MBBR and FBBR are shown in Table S1.
Sample preparation and atrazine and COD analysis
To analyze atrazine and COD, a 50 mL sample was collected from each bioreactor followed by filtration using Whatman-45 (0.4) μm and storage in a refrigerator at 4°C; the amount of sample needed for COD and HPLC analysis is 2.5 mL and 100 μL, respectively. Analytical analyses were performed in less than 12 h of sample collection. Atrazine in the effluent was analyzed with HPLC-UV spectroscopy (MACHEREY-NAGEL, nucleodur) equipped with S 2500 sample injector, S 2100 solvent delivery system, S 3210 UV/ Vis detector, S 4011 column thermo controller, and EC 250/4.6 Nucleodur 100-5 C18ec column. The mobile phase includes methanol: water (70:30, v/v) with a flow rate of 0.7 mL/min. In addition, The HPLC sample volume was set at 10 μL. In addition, the UV detection wavelength used was 230 nm according to Jacomini, et al. [45]. Under these conditions, atrazine peaked at 3.4 min. Moreover, the quantities of COD in effluent were carried out according to standard methods using a DR-5000 spectrophotometer [46].
Atrazine removal kinetics
Kinetic analysis is used to explain and predict the performance of biological reactors in contaminant removal from wastewater [47]. Biological models have been developed to assess and optimize the empirical design, to describe the relationship between vital parameters (i.e. volume, HRT, influent, and effluent concentrations of the substrate), and to predict and monitor bioreactor performance. The modified Stover-Kincannon model has proven to have the highest degree of precision in predicting bioreactor performance in atrazine removal [27]. Therefore, we used the modified Stover-Kincannon model to investigate the kinetics of atrazine removal by MBBR and FBBR.
In the modified Stover-Kincannon model, the organic removal rate for biofilm reactors is a function of the organic loading rate as shown in Equation (1) [48].
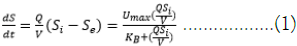
Where, dS/dt is the rate at which the concentration of a substrate is decreasing over time 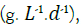 Q is the influent flow rate (L. d-1), V is bioreactor volume (L), S0 is the substrate concentration at the influent
Q is the influent flow rate (L. d-1), V is bioreactor volume (L), S0 is the substrate concentration at the influent 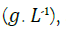 and S is the substrate concentration at the effluent
and S is the substrate concentration at the effluent 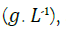 Umax is maximum utilization rate constant
Umax is maximum utilization rate constant 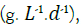 and KB is saturation value constant
and KB is saturation value constant 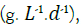 .
.
Linearization of Equation (1), results in Equation (2) as follows:
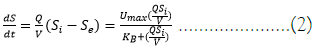
Plotting V/Q(Si−Se) against V/QSi yield in a straight line with slope (gradient) and intercept equal to KB/Umax and 1/Umax, respectively.
Results and Discussion
Effect of initial atrazine concentration on Atrazine biodegradation
The removal efficiency of atrazine from wastewater depends on various factors, including atrazine concentration at the influent. In this study, the effectiveness of MBBR and FBBR in atrazine removal from wastewater was evaluated at various initial concentrations of atrazine including 5, 10, 15, 20, and 30 mg/L. Figure 2, indicates the atrazine removal efficiency in MBBR and FBBR at different initial concentrations under a constant nutrient ratio equal to COD:N:P=100:5:1. In general, the atrazine removal efficiency increased as the initial concentrations increased. For example, when the initial concentrations of atrazine were 5 mg/L, at an HRT of 5 h, the elimination of atrazine in MBBR and FBBR was 22.2% and 27.1%, respectively, and it reached 55.8% and 62.0% at the concentration of 30 mg/L. In addition, at an HRT of 10 hours, atrazine removal efficiency for initial concentrations of atrazine at 5, 10, 15, 20, and 30 mg/L was 28.0%, 33.9%, 42.4%, 52.3%, and 67.0% in MBBR and 34.4%, 37.8%, 45.4%, 56.5%, and 74.2% in FBBR. At an HRT equal to 24 h, the atrazine elimination efficiency in MBBR and FBBR was 33.1% and 39.6% at initial atrazine concentrations of 5 mg/L and it reached 75.2% and 81.6% at the concentration of 30 mg/L, respectively.
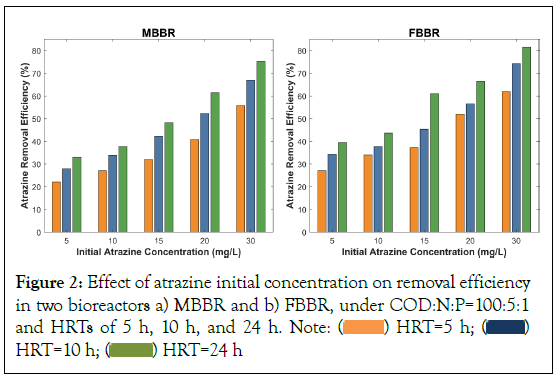
Figure 2: Effect of atrazine initial concentration on removal efficiency
in two bioreactors a) MBBR and b) FBBR, under COD:N:P=100:5:1 and HRTs of 5 h, 10 h, and 24 h. Note:  HRT=10 h;
HRT=10 h;  HRT=24 h
HRT=24 h
These results are consistent with previous studies investigating biological atrazine removal from wastewater [23,26,31]. For example, Baghapour, et al. [31], assessed atrazine removal using a submerged biological aerated filter and observed an increase in higher removal efficiency at higher initial concentrations of atrazine, reaching maximum efficiency of 97.9% under the initial concentrations at 10 mg/L (highest initial concentrations) and HRT of 24 h [31]. The same trend between initial atrazine concentrations and removal efficiency has been observed in fixed bed sequence batch reactors using a volcanic pumice stone [26]. In some studies, the atrazine removal efficiency decreased immediately after increasing atrazine concentrations due to the toxic shock effect caused by the increased atrazine levels, as well as the potential inhibitory impacts of atrazine on the degrading activities of microorganisms. However, subsequently, there was a gradual improvement in bioreactor performance, indicating an ascending trend. This improvement can be attributed to the microbial acclimatization to the presence of atrazine [25,27,28]. Higher concentrations of atrazine may increase its accessibility to microorganisms, resulting in increased consumption and removal efficiency of atrazine at higher initial concentrations once the microbial consortium was acclimatized to the toxic substance in the environment.
Effect of retention time on atrazine biodegradation
Retention time can have a significant effect on the biological removal of atrazine. To assess the effect of retention time on biological atrazine removal, three different retention times were selected (5, 10, and 24 h). Figure 3, indicates the impact of different HRT on atrazine removal efficiency in MBBR and FBBR. Based on our results, both bioreactors exhibited an overall increase in atrazine removal as HRT increased. For example, at an initial atrazine concentration of 5 mg/L and COD:N:P=100:5:1, atrazine removal efficiency in MBBR and FBBR were estimated as 22.2% and 27.1%, respectively, while it reached 28.0%% and 34.4% at HRT of 10 h, and 33.1% and 39.6% at HRT of 24 h. In addition, at initial concentrations of 30 mg/L, removal efficiency at HRT of 5, 10, and 24 were 55.8%, 67.0%, and 75.2% in MBBR, and 62.0%, 74.2%, and 81.6% in FBBR, respectively. Overall, at HRTs of 5 h, 10 h, and 24 h atrazine removal efficiency was 33.1%, 42.9%, and 49.3% in MBBR, and 40.2%, 48.0%, and 56.7% in FBBR, respectively, highlighting that atrazine removal efficiency increased with longer HRTs.
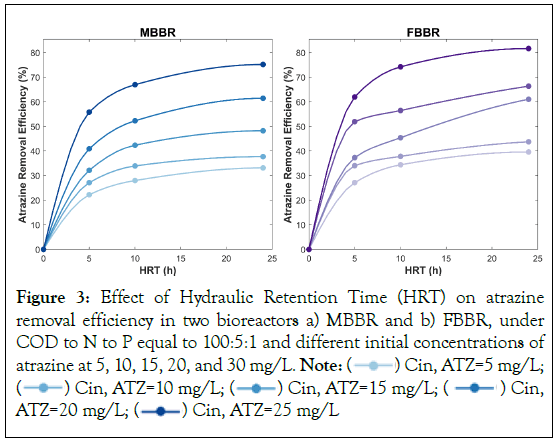
Figure 3: Effect of Hydraulic Retention Time (HRT) on atrazine
removal efficiency in two bioreactors a) MBBR and b) FBBR, under
COD to N to P equal to 100:5:1 and different initial concentrations of atrazine at 5, 10, 15, 20, and 30 mg/L. Note:  Cin, ATZ=5 mg/L;
Cin, ATZ=5 mg/L;  Cin, ATZ=20 mg/L;
Cin, ATZ=20 mg/L;  Cin, ATZ=25 mg/L
Cin, ATZ=25 mg/L
Studies have shown that longer retention times can lead to increased atrazine removal rates in biological treatment systems [23-31]. For example, one study found that increasing HRT from 6 to 12 h in a laboratory-scale bioreactor resulted in a significant increase in atrazine removal efficiency from 32% to 72% [27]. This trend can be explained by the fact that longer retention times provide more time for the atrazine to come into contact with the microorganisms in the system that degrade atrazine. As the microorganisms have more time to interact with the atrazine, they can break it down more effectively, leading to higher removal efficiency. Despite the increase in atrazine removal efficiency with higher HRT, our results indicate that the rate of removal was faster during the first five hours of operation in both MBBR and FBBR and then gradually slowed down as the retention time increased. This suggests that while longer retention times can improve atrazine removal efficiency, there may be diminishing returns beyond a certain point.
Effect of nitrogen concentrations on atrazine biodegradation
Studies have shown that the availability of nutrient ratios may impact the biological removal of atrazine [30,39]. To assess the impact of nitrogen on atrazine biodegradability, we conducted experiments under different COD:N:P ratios, with a ratio of 100:5:1 and 100:15:1. Figure 4, indicates the impact of nitrogen concentrations on the efficiency of MBBR and FFBR in atrazine removal. Increasing nitrogen concentrations generally resulted in a decrease in atrazine removal efficiency in both bioreactors by up to 11.3%. Under COD:N:P ratio equal to 100:5:1, the atrazine removal efficiency was 43.8% and 50.2% in MBBR and FBBR, respectively; while under COD:N:P ratio equal to 100:15:1, the removal efficiency was slightly lower, with 39.7% and 46.4% in MBBR and FBBR, respectively. The results of our study indicate a decrease in atrazine biodegradation at higher nitrogen levels, which is consistent with previous studies [30]. Most atrazine-degrading bacteria utilized atrazine as a nitrogen source [41]. Therefore, many studies focused on the impacts of nitrogen compounds on atrazine catabolism, and they reported that nitrogen amendments led to a decrease in atrazine biodegradation by the indigenous microbial population in soil [40], pure culture [41], and aquatic environments [39]. For example, atrazine removal efficiency from water by biobarriers was reduced by 60% when nitrate was added to the water compared to nitrogen-limiting conditions [39].
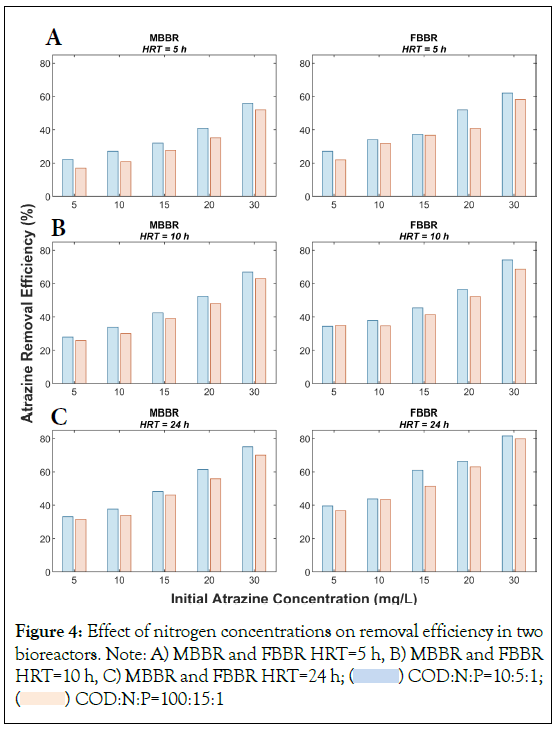
Figure 4: Effect of nitrogen concentrations on removal efficiency in two
bioreactors. Note: A) MBBR and FBBR HRT=5 h, B) MBBR and FBBR HRT=10 h, C) MBBR and FBBR HRT=24 h;  COD:N:P=10:5:1;
COD:N:P=10:5:1;  COD:N:P=100:15:1
COD:N:P=100:15:1
Kinetic coefficients
The biological model provides a useful tool for predicting the removal of atrazine in bioreactor systems, and can be used to design and optimize these systems for wastewater treatment applications. The use of the modified Stover-Kincannon model to determine the kinetics of atrazine biodegradation has been reported in several studies, and it has been shown to provide the highest degrees of precision in predicting the rate of atrazine removal [24,25]. Therefore, we have applied modified Stover-Kincannon to assess the kinetics of atrazine removal in FBBR and MBBR. Under the nutrient ratio (COD:N:P) equal to 100:5:1, the KB and Umax constants were computed equal to 4.15 and 1.49ATZ L-1.d-1 in MBBR, and 5.73 and 2.30ATZ L-1.d-1 in FBBR as shown as Figure 5.
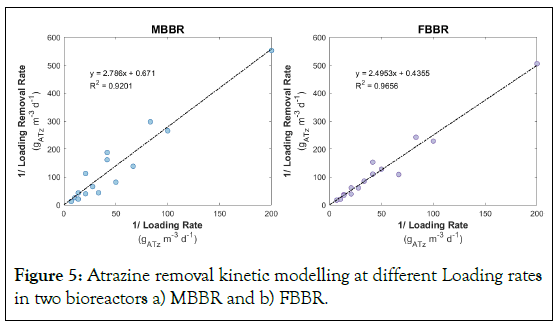
Figure 5: Atrazine removal kinetic modelling at different Loading rates in two bioreactors a) MBBR and b) FBBR.
Performance comparison of MBBR and FBBR in atrazine removal
Our results indicate that both the FBBR and MBBR technologies are effective in removing atrazine and COD from wastewater, although the specific performance varies depending on the specific operating conditions. Comparing the two treatment systems, it seems that FBBR had a higher atrazine removal efficiency than MBBR in removing both atrazine and COD. Depending on atrazine initial concentrations, HRT, and nutrient ratio, the removal efficiency of the FBBR system ranged from 17.0% to 75.2% with an average removal efficiency of 41.8%, while the MBBR system had minimum, average, and maximum removal efficiency of 21.9%, 48.3%, and 81.6%, respectively. Based on the information provided in Table 1, the FBBR appears to have a higher Umax and KB compared to the MBBR, indicating that FBBR can potentially treat atrazine more efficiently. Additionally, the FBBR also has a higher R2 value, indicating that the Stover- Kincannon equation fits the experimental data better. FBBR also performed better in COD removal, with an average removal efficiency of 72.9%, while MBBR achieved 63.4%. Our findings are consistent with prior studies evaluating the performance of MBBR and FBBR in contaminant removal. For example, Choi, et al. [49], observed that FBBR outperformed MBRR in biological phosphate removal and denitrification and was more effective in removing COD, Biochemical Oxygen Demand (BOD), acetate, phosphate, and nitrate nitrogen compared to MBBR [49]. Another factor to consider is the operational complexity and cost of the two systems. The FBBR system typically requires more maintenance and monitoring due to the need to control biomass retention, while the MBBR system is generally considered to be simpler to operate [50,51].
| Bioreactors | Umax | KB | R2 | Stover–Kincannon equation |
|---|---|---|---|---|
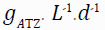 |
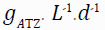 |
|||
| MBBR | 1.49 | Jan-00 | 0.9201 | 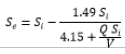 |
| FBBR | 2.3 | 5.73 | 0.9656 | 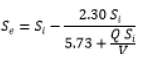 |
Table 1: Stover–Kincannon model and coefficients for atrazine removal in MBBR and FBBR.
Conclusion
The study investigated FBBR and MBBR in removing atrazine from low-strength wastewater to evaluate the efficiency of these two technologies for atrazine removal and their potential application as a cost-effective and sustainable treatment option. To assess the impacts of environmental conditions on atrazine biodegradability, tests were conducted at different initial atrazine concentrations (5, 10, 15, 20, and 30 mg/L), different HRTs (5, 10, and 24 h), and different nutrient ratio (COD:N:P) equal to 100:5:1 and 100:15:1. Moreover, all experiments were conducted with COD of 200 mg/L to evaluate bioreactor effectiveness in removing atrazine from low-strength wastewater. In addition, we have applied the modified Stover-Kincannon model to investigate the kinetics of atrazine removal by MBBR and FBBR. Based on the given results, it appears that both the FBBR and MBBR technologies are effective in removing atrazine and COD from wastewater, although the specific performance varies depending on the specific operating conditions. Atrazine removal efficiency of FBBR was higher than MBBR in removing both atrazine and COD. The average COD removal efficiency in MBRR and FBBR is 63.4% and 72.9%, respectively. The average and maximum atrazine removal efficiency was 41.8% and 75.2% in MBBR, and 48.3% and 81.6% in FBBR, respectively. A reduction in atrazine removal was observed with higher nitrogen levels in FBBR and MBBR. Higher HRTs and initial atrazine concentrations increased atrazine removal efficiency in both bioreactors. Under the nutrient ratio equal to COD:N:P ratio of 100:5:1, the values of KB and Umax constants were modeled to be 4.15 and 1.49 gATZ L-1.d-1 in MBBR, and 5.73 and 2.30 gATZ L-1.d-1 in FBBR. This study provided valuable insights into the potential of bioreactors as a sustainable technology for atrazine removal, leading to the development of efficient and cost-effective wastewater treatment strategies.
Acknowledgments
We would like to thank the peer reviewers for their valuable feedback and suggestions. We also extend our gratitude to Zahra Ghobadi Nejad from the Biochemical and Bioenvironmental Research Center at Sharif University for her insightful contributions to this work.
Declaration of Competing Interest
The authors do not have any relevant competing interests to disclose in relation to the content of this article.
References
- Lassere JP, Fack F, Revets D, Renaut J, Bohn T, Gutleb AC, et al. Effects of the endocrine disrupting compounds atrazine and PCB 153 on the protein expression of MCF-7 human breast cancer cells. J Proteome Res. 2009;8(12):5485-5496.
[Crossref] [Google Scholar] [PubMed]
- Rostami S, Jafari S, Moeini Z, Jaskulak M, Keshtgar L, Badeenezhad A, et al. Current methods and technologies for degradation of atrazine in contaminated soil and water: A review. Environ Technol Inn. 2021;24:102019.
- deSouza RM, Seibert D, Quesada HB, Bassetti FDJ, Fagundes-Klen MR, Bergamasco R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf Environ Prot. 2020;135:22-37.
- Wang A, Hu X, Wan Y, Mahai G, Jiang Y, Huo W, et al. A nationwide study of the occurrence and distribution of atrazine and its degradates in tap water and groundwater in China: Assessment of human exposure potential. Chemosphere. 2020;252:126533.
[Crossref] [Google Scholar] [PubMed]
- Yu H, Liu Y, Shu X, Fang H, Sun X, Pan Y, et al. Equilibrium, kinetic and thermodynamic studies on the adsorption of atrazine in soils of the water fluctuation zone in the Three-Gorges Reservoir. Environmental Sciences Europe. 2020;32(1):1-10.
- Carter A. How pesticides get into water-and proposed reduction measures. Pestic Outlook. 2000;11(4):149-156.
- Vryzas Z. Pesticide fate in soil-sediment-water environment in relation to contamination preventing actions. Curr Opin Environ Sci Health. 2018;4:5-9.
- Barchanska H, Babilas B, Gluzicka K, Zralek D, Baranowska I. Rapid determination of mesotrione, atrazine and its main degradation products in selected plants by MSPD–HPLC and indirect estimation of herbicides phytotoxicity by chlorophyll quantification. J Environ Anal Chem. 2014;94(2):99-114.
- Chang J, Fang W, Chen L, Zhang P, Zhang G, Zhang H, et al. Toxicological effects, environmental behaviors and remediation technologies of herbicide atrazine in soil and sediment: A comprehensive review. Chemosphere. 2022;307(Pt3):136006.
[Crossref] [Google Scholar] [PubMed]
- Jablonowski ND, Schäffer A, Burauel P. Still present after all these years: Persistence plus potential toxicity raise questions about the use of atrazine. Environ Sci Pollut Res Int. 2011;18:328-331.
[Crossref] [Google Scholar] [PubMed]
- Agopian A, Lupo PJ, Canfield MA, Langlois PH. Case-control study of maternal residential atrazine exposure and male genital malformations. Am J Med Genet A. 2013;161A(5):977-982.
[Crossref] [Google Scholar] [PubMed]
- Almasi H, Takdastan A, Jaafarzadeh N, Babaei AA, Birgani YT, Cheraghian B, et al. Spatial distribution, ecological and health risk assessment and source identification of atrazine in Shadegan international wetland, Iran. Mar Pollut Bull. 2020;160:111569.
- Pathak RK, Dikshit AK. Atrazine and human health. Int J Ecosyst. 2011;1(1):14-23.
- Elgueta S, Correa A, Campo M, Gallardo F, Karpouzas D, Diez MC. Atrazine, chlorpyrifos, and iprodione effect on the biodiversity of bacteria, actinomycetes, and fungi in a pilot biopurification system with a green cover. J Environ Sci Health B. 2017;52(9):651-657.
[Crossref] [Google Scholar] [PubMed]
- Lin J, Li HX, Qin L, Du ZH, Xia J, Li JL. A novel mechanism underlies atrazine toxicity in quails (Coturnix coturnix): Triggering ionic disorder via disruption of ATPases. Oncotarget. 2016;7(51):83880.
[Crossref] [Google Scholar] [PubMed]
- Fairchild J. Structural and functional effects of herbicides on non-target organisms in aquatic ecosystems with an emphasis on atrazine. Herbicide Env. 2011;383-404.
- Rohr JR, McCoy KA. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ Health Perspect. 2010;118(1):20-32.
[Crossref] [Google Scholar] [PubMed]
- Hayes TB, Anderson LL, Beasley VR, De Solla SR, Iguchi T, Ingraham H, et al. Demasculinization and feminization of male gonads by atrazine: Consistent effects across vertebrate classes. J Steroid Biochem Mol Biol. 2011;127(1-2):64-73.
[Crossref] [Google Scholar] [PubMed]
- Horzmann KA, Reidenbach LS, Thanki DH, Winchester AE, Qualizza BA, Ryan GA, et al. Embryonic atrazine exposure elicits proteomic, behavioral, and brain abnormalities with developmental time specific gene expression signatures. J Proteomics. 2018;186:71-82.
[Crossref] [Google Scholar] [PubMed]
- Saleh IA, Zouari N, Al-Ghouti MA. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ Technol Inn. 2020;19:101026.
- Ghosh PK, Philip L. Environmental significance of atrazine in aqueous systems and its removal by biological processes: An overview. Glob NEST J. 2006;8(2):159-178.
- Jatoi AS, Hashmi Z, Adriyani R, Yuniarto A, Mazari SA, Akhter F, et al. Recent trends and future challenges of pesticide removal techniques-A comprehensive review. J Environ Chem Eng. 2021;9(4):105571.
- Baghapour MA, Nasseri S, Derakhshan Z. Atrazine removal from aqueous solutions using submerged biological aerated filter. J Environ Health Sci Eng. 2013;11:1-9.
[Crossref] [Google Scholar] [PubMed]
- Derakhshan Z, Ehrampoush MH, Mahvi AH, Dehghani M, Faramarzian M, Eslami H. A comparative study of hybrid membrane photobioreactor and membrane photobioreactor for simultaneous biological removal of atrazine and CNP from wastewater: A performance analysis and modeling. J Chem Eng. 2019;355:428-438.
- Derakhshan Z, Ehrampoush MH, Mahvi AH, Dehghani M, Faramarzian M, Ghaneian MT, et al. Evaluation of a moving bed biofilm reactor for simultaneous atrazine, carbon and nutrients removal from aquatic environments: Modeling and optimization. J Ind Eng Chem. 2018a;67:219-230.
- Derakhshan Z, Ehrampoush MH, Mahvi AH, Faramarzian M, Mokhtari M, Mazloomi SM. Evaluation of volcanic pumice stone as media in fixed bed sequence batch reactor for atrazine removal from aquatic environments. Water Sci Technol. 2016;74(11):2569-2581.
[Crossref] [Google Scholar] [PubMed]
- Derakhshan Z, Ehrampoush MH, Mahvi AH, Ghaneian MT, Mazloomi SM, Faramarzian M, et al. Biodegradation of atrazine from wastewater using moving bed biofilm reactor under nitrate-reducing conditions: A kinetic study. J Environ Manage. 2018b;212:506-513.
[Crossref] [Google Scholar] [PubMed]
- Derakhshan Z, Mahvi AH, Ehrampoush MH, Mazloomi SM, Faramarzian M, Dehghani M, et al. Studies on influence of process parameters on simultaneous biodegradation of atrazine and nutrients in aquatic environments by a membrane photobioreactor. Environ Res. 2018c;161:599-608.
[Crossref] [Google Scholar] [PubMed]
- Derakhshan Z, Mahvi AH, Ghaneian MT, Mazloomi SM, Faramarzian M, Dehghani M, et al. Simultaneous removal of atrazine and organic matter from wastewater using anaerobic moving bed biofilm reactor: A performance analysis. J Environ Manage. 2018d;209:515-524.
[Crossref] [Google Scholar] [PubMed]
- Hassanpour B, Geohring LD, Klein AR, Giri S, Aristilde L, Steenhuis TS. Application of denitrifying bioreactors for the removal of atrazine in agricultural drainage water. J Environ Manage. 2019;239:48-56.
[Crossref] [Google Scholar] [PubMed]
- Nasseri S, Baghapour MA, Derakhshan Z, Faramarzian M. Degradation of atrazine by microbial consortium in an anaerobic submerged biological filter. J Water Health. 2014;12(3):492-503.
[Crossref] [Google Scholar] [PubMed]
- Li XK, Ji WJ, Zhao J, Wang SJ, Au CT. Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. J Catal. 2005;236(2):181-189.
- Kim Y, Li S, Chekli L, Woo YC, Wei C-H, Phuntsho S, et al. Assessing the removal of organic micro-pollutants from anaerobic membrane bioreactor effluent by fertilizer-drawn forward osmosis. J Membr Sci. 2017;533:84-95.
- Lopes TSDA, Hessler R, Bohner C, Junior GBA, de Sena RF. Pesticides removal from industrial wastewater by a membrane bioreactor and post-treatment with either activated carbon, reverse osmosis or ozonation. J Environ Chem Eng. 2020;8(6):104538.
- Carneiro RB, Mukaeda CM, Sabatini CA, Santos-Neto ÁJ, Zaiat M. Influence of organic loading rate on ciprofloxacin and sulfamethoxazole biodegradation in anaerobic fixed bed biofilm reactors. J Environ Manage. 2020;273:111170.
[Crossref] [Google Scholar] [PubMed]
- Schlegel S, Koeser H. Wastewater treatment with submerged fixed bed biofilm reactor systems-design rules, operating experiences and ongoing developments. Water Sci Technol. 2007;55(8-9):83-89.
[Crossref] [Google Scholar] [PubMed]
- Bjornberg C, Lin W, Zimmerman R. Effect of temperature on biofilm growth dynamics and nitrification kinetics in a full-scale MBBR system. WEF. 2009:4407-4426.
- Thakur SA, Khedikar IP. Performance evaluation of Moving Bed Bio-Film Reactor (MBBR) for treatment of domestic wastewater. Int J Sci Res. 2015;6(4):973-976.
- Hunter WJ, Shaner DL. Nitrogen limited biobarriers remove atrazine from contaminated water: Laboratory studies. J Contam Hydrol. 2009;103(1-2):29-37.
[Crossref] [Google Scholar] [PubMed]
- Garcés RAG, Hansen AM, Van Afferden M. Mineralization of atrazine in agricultural soil: Inhibition by nitrogen. Environ Toxicol Chem. 2007;26(5):844-850.
[Crossref] [Google Scholar] [PubMed]
- García-González V, Govantes F, Shaw LJ, Burns RG, Santero E. Nitrogen control of atrazine utilization in Pseudomonas sp. strain ADP. Appl Environ Microbiol. 2003;69(12):6987-6993.
[Crossref] [Google Scholar] [PubMed]
- Crone BC, Garland JL, Sorial GA, Vane LM. Significance of dissolved methane in effluents of anaerobically treated low strength wastewater and potential for recovery as an energy product: A review. Water res. 2016;104:520-531.
[Crossref] [Google Scholar] [PubMed]
- Singh KS, Harada H, Viraraghavan T. Low-strength wastewater treatment by a UASB reactor. Bioresour Technol. 1996;55(3):187-194.
- Evy A, Lakshmi V, Nilanjana D. Biodegradation of atrazine by Cryptococcus laurentii isolated from contaminated agricultural soil. J Microbiol Biotechnol Res. 2012;2(3):450-457.
- Jacomini AE, Bonato PS, Avelar WEP. HPLC method for the analysis of atrazine in freshwater bivalves. J Liq Chromatogr R T. 2003;26(12):1885-1894.
- Apha A. Standard methods for the examination of water and wastewater. APHA, AWWA, WEF. 2007.
- Hassani AH, Borghei SM, Samadyar H, Ghanbari B. Utilization of moving bed biofilm reactor for industrial wastewater treatment containing ethylene glycol: Kinetic and performance study. Environ Technol. 2014;35(4):499-507.
[Crossref] [Google Scholar] [PubMed]
- Borghei S, Sharbatmaleki M, Pourrezaie P, Borghei G. Kinetics of organic removal in fixed-bed aerobic biological reactor. Bioresour Technol. 2008;99(5):1118-1124.
[Crossref] [Google Scholar] [PubMed]
- Choi H, Lee A, Lee S. Comparison between a moving bed bioreactor and a fixed bed bioreactor for biological phosphate removal and denitrification. Water Sci Technol. 2012;65(10):1834-1838.
[Crossref] [Google Scholar] [PubMed]
- Jiang H, Zhang Z, Lin Z, Gong X, Guo H, Wang H. Modification of polyurethane sponge filler using medical stones and application in a moving bed biofilm reactor for ex situ remediation of polluted rivers. J Water Process Eng. 2021;42:102189.
- Lariyah M, Mohiyaden H, Hayder G, Hussein A, Basri H, Sabri A, et al. Application of Moving Bed Biofilm Reactor (MBBR) and Integrated Fixed Activated Sludge (IFAS) for biological river water purification system: A short review. IOP Conf Ser Earth Environ Sci. 2016;012005.
Citation: Kamanmalek S, Rahmatabad AD, Borghei SM (2024) Biological Atrazine Removal from Low-Strength Wastewater by Moving Bed Biofilm Reactor and Upflow Fixed Bed Bioreactor: Performance and Kinetic Analysis. Glob J Agric Health Sci. 13:226.
Copyright: © 2024 Kamanmalek S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.