Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 15, Issue 2
Bioequivalence Study of Rosuvastatin 20 mg Tablets in Healthy Thai Volunteers under Fasting Conditions
Jaturavit Vattanarongkup1, Charinthon Seeduang1, Sumate Kunsa-ngiem1, Vipada Khaowroongrueng1*, Lalinthip Saeaue1, Busarat Karachot1, Piengthong Narakorn1, Porranee Puranajoti2 and Isariya Techatanawat12International Bio Service Co., Ltd., 888 Golden Jubilee Medical Center, Mahidol University, Nakhon Pathom 73170, Thailand
Received: 28-Feb-2023, Manuscript No. JBB-23-20024; Editor assigned: 03-Mar-2023, Pre QC No. JBB-23-20024 (PQ); Reviewed: 17-Mar-2023, QC No. JBB-23-20024; Revised: 24-Mar-2023, Manuscript No. JBB-23-20024 (R); Published: 31-Mar-2023, DOI: 10.35248/0975-0851.23.15.508
Abstract
Rosuvastatin is a member of high-potency statins. It is indicated for the treatment of homozygous familial hypercholesterolemia, hyperlipidemia, mixed dyslipidemia, primary dysbetalipoproteinemia, and hypertriglyceridemia. The study aimed to compare the rate and extent of absorption as well as safety of two rosuvastatin 20 mg tablet formulations; the test product ROZACOR® manufactured by the Government Pharmaceutical Organization, Thailand and the reference product CRESTOR® manufactured by IPR Pharmaceuticals Inc., Puerto Rico. An open label, randomized, two-treatment, two-sequence, two-period, single oral dose, crossover bioequivalence study was conducted in healthy Thai adult volunteers under fasting conditions. The concentrations of rosuvastatin in plasma samples were determined using a validated liquid chromatography-tandem mass spectroscopy method. The pharmacokinetic parameters were computed from the plasma concentration-time data using a non-compartmental model. The 90% confidence intervals of the geometric least squares mean ratios (test/reference) were 91.81%- 104.50%, 93.26%-105.29% and 89.88%-105.96% for ln-transformed AUC0-tlast, AUC0-∞ and Cmax, respectively which were within the bioequivalence limits of 80.00%-125.00%. Both test and reference products were well tolerated by the study subjects. There were total 5 incidents of mild adverse events reported in this study. In conclusion, ROZACOR® and CRESTOR® were bioequivalent in terms of rate and extent of absorption and can be used interchangeably.
Keywords
Rosuvastatin; Bioequivalence; Pharmacokinetics; Hyperlipidemia
Introduction
Hyperlipidemia is a term used to describe elevated low-density lipoprotein cholesterol, total cholesterol, triglycerides, or both total cholesterol and triglycerides levels in the blood [1]. Patients with hyperlipidemia also carry the risk of cardiovascular disease including myocardial infarction and stroke leading to death and disability worldwide. Statin, lipid-lowering agent has been demonstrated to reduce risk of morbidity and mortality in patients [2-3].
Rosuvastatin which is a member of high-potency statins has been approved by the U.S. FDA since August 2003. Like other statins, it inhibits the enzyme 3-Hydroxy-3-Methylglutaryl CoA (HMG-CoA) reductase, which is a rate-limiting step in cholesterol biosynthesis. It is thus indicated to reduce elevated levels of low-density lipoprotein cholesterol, total cholesterol and triglycerides in patients with homozygous familial hypercholesterolemia, hyperlipidemia, mixed dyslipidemia, primary dysbetalipoproteinemia, and hypertriglyceridemia [4-6]. At daily dose of 5-40 mg, it has shown 45% to 63% reduction in low-density lipoprotein cholesterol, which is statistically greater than atorvastatin, simvastatin, and pravastatin at equivalent dose. Moreover, rosuvastatin is also known to be effective in increasing high-density lipoprotein cholesterol levels [7]. Peak plasma concentration of rosuvastatin is achieved within 3-5 hours after oral administration. The absolute oral bioavailability is approximately 20%. Food may delay the rate of absorption while the extent of absorption is unaffected [8]. Rosuvastatin is 88% bound to plasma proteins, mostly to albumin. It is primarily metabolized to an N-desmethyl rosuvastatin by cytochrome P450 2C9 and 2C19. Approximately 90% of the administered dose is eliminated by excretion in the feces as unchanged form. The elimination half- life of rosuvastatin is approximately 19 hours [9-10]. Rosuvastatin exhibits linear pharmacokinetics over the dose range of 5-80 mg [11].
In Thailand, hyperlipidemia is one of the major risk factors for coronary heart disease [12]. The Government Pharmaceutical Organization (GPO), Thailand had developed a generic product of rosuvastatin 20 tablets, ROZACOR® as an alternative choice for physicians and patients with the aim of improving accessibility to medicines for Thai patients. To ensure that the generic product, (ROZACOR®) maintains the same quality and safety as the reference product (CRESTOR®), the bioequivalence study was conducted in healthy Thai volunteers to demonstrate the equivalence in biopharmaceutics quality between the generic and reference medicinal product.
Materials and Methods
Study products
Rosuvastatin 20 mg tablets were compared between the test product, ROZACOR® (Lot No. S645019), manufactured by GPO, Thailand and the reference product, CRESTOR® (Lot No. 60039082), manufactured by IPR Pharmaceuticals Inc., Puerto Rico and imported by AstraZeneca (Thailand) Ltd., Bangkok, Thailand.
Study subject
The sample size was computed based on the maximum intra-subject variability for Cmax of rosuvastatin about 28.5%, T/R ratio at 95%, significance level at 5%, and bioequivalence limits of 80.00%- 125.00% [13-14]. According to the calculation, the sample size of 40 study subjects were sufficient for establishing bioequivalence with the power greater than 85%. However, 50 study subjects were enrolled in the study considering 20% dropouts and withdrawals.
A total of 50 healthy male and female subjects between 18-55 years of age, with a Body Mass Index (BMI) between 18.0-30.0 kg/m2, were enrolled in the study. All subjects were determined healthy based on the medical history, physical and laboratory examinations including complete blood count, hematocrit, hemoglobin, fasting blood sugar, blood urea nitrogen, serum creatinine, creatine phosphokinase, creatinine clearance, alkaline phosphatase, ALT, AST, total bilirubin, total protein albumin, HBsAg, Anti-HCV, Anti-HIV, urine analysis, chest X-ray and 12-Lead ECG.
The participants who were contraindicated or hypersensitive to rosuvastatin or any excipients in the formulations were excluded. Additional exclusion criteria for this study included history or presence of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption, evidence of underlying disease, illness within 4 weeks before the start of the study, participation in any other clinical trials, recent blood donation and history of drug abuse. Subjects were restricted from smoking, any medication (including over-the-counter products, herbal remedies), vitamins, or dietary supplements, grapefruit/pomelo/orange-based products, xanthine containing products, and alcohol prior to and during study participation. Subjects were well informed of the studies and gave their consent before study participation.
Study design
An open label, randomized, two-treatment, two-sequence, two- period, single oral dose, crossover study was conducted under fasting conditions. Fifty study subjects were randomly assigned to two groups, Test-Reference (TR) and Reference-Test (RT) according to the randomization schedule of receiving the product in each period of the study. After at least 10 hours overnight fasting, a single tablet of either the test or reference formulation was administered with 240 mL of water, followed by mouth and hand check to assess dosing compliance. A standardized lunch was served at 4 hours after drug administration. After a washout period of 14 days, the procedure was repeated in the same manner to complete the crossover design. Adverse events were monitored throughout the study based on direct questioning, clinical and laboratory examinations. The study was approved by The Institute for the Development of Human Research Protections (IHRP), Department of Medical Sciences, Ministry of Public Health, Thailand, and was conducted as per the protocol in compliance with ‘Guideline for Good Clinical Practice’ of The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), Declaration of Helsinki, and European Medicines Agency (EMA) guideline on the investigation of bioequivalence [15]. The clinical activities were performed at International Bio Service Co., Ltd., Golden Jubilee Medical Center, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand.
Blood sampling
Serial blood samples were collected at pre-dose (0 hour), 0.33, 0.67, 1, 1.33, 1.67, 2, 2.33, 2.67, 3, 3.33, 3.67, 4, 4.33, 4.67, 5, 5.5, 6, 7, 8, 10, 12, 16, 24, 34, 48 and 72 hours after oral administration in each period through an indwelling intravenous cannula placed in a forearm vein of the subject. The samples were transferred into collection tube containing dipotassium ethylenediaminetetraacetate (K2EDTA) as the anticoagulant and placed in wet ice water bath before centrifugation and during separation. The plasma samples were prepared from centrifugation at 3000 ± 100 rcf for 5 minutes at below 10°C. Each plasma sample was transferred into tube containing buffer solution (20 mM ammonium formate : 100 mM sodium fluoride : formic acid (45:45:10 v/v)) at 2% of total plasma volume. The buffered plasma samples were stored at -50°C or colder until sample analysis at Bioequivalence Study group, Research and Development Institute, The Government Pharmaceutical Organization (GPO), Thailand.
Study sample analysis
The concentrations of rosuvastatin in human plasma samples were determined using validated liquid chromatography-tandem mass spectroscopy (LC-MS/MS) methods over a concentration range of 0.102 to 70.028 ng/mL. For sample preparation, 250 µL of plasma was processed using liquid-liquid extraction technique by adding 1% formic acid solution (v/v) and diethyl ether: hexane (80:20, v/v) solution into each sample. Rosuvastatin-d6 was used as an internal standard. The samples were centrifuged at 3400 ± 100 rcf for 5 minutes at 10°C, and then flash-frozen to separate the organic layer. The organic layer was subsequently evaporated at 40°C to dryness and reconstituted with acetonitrile: water (80:20, v/v) solution. The samples were injected at 5 µL into a chromatographic system consisting of ACQUITY UPLC® BEH C18 1.7 µm 2.1 × 50 mm column,and ExionLC™ system coupled with Triple Quad® 4500 (AB Sciex, Singapore). A gradient elution was carried out by changing the composition of 0.05% formic acid in water solution (v/v) and 0.05% formic acid in acetonitrile solution (v/v) at flow rate of 0.4 mL/minute. Mass spectrometer will be operated in a direct flow mode and monitored in the positive ion mode using electrospray ionization and following ion transitions; m/z 482.200 → m/z 258.100 for rosuvastatin and m/z 488.300 → m/z 264.300 for rosuvastatin-d6. Chromatographic data was processed by Analyst® version 1.7.0 (AB Sciex, Singapore).
According to EMA guideline on bioanalytical method validation, study samples at the concentrations close to maximum concentration and in the elimination phase of each subject in each period were chosen for incurred sample reanalysis [16]. However, the concentrations of incurred samples were not used for pharmacokinetic calculation.
Pharmacokinetic and statistical analysis
The pharmacokinetic parameters calculated from plasma concentration-time data using a non-compartmental model of Phoenix WinNonlin Software Version 6.4 (Pharsight Corporation, USA). Maximum concentration (Cmax) and time to maximum concentration (tmax) were obtained directly from the data. Area under the plasma concentration-time curve from time zero to the last measurable concentration (AUC0-tlast) was calculated by linear trapezoidal method. Area under the plasma concentration- time curve from time zero to infinity (AUC0-∞) was calculated as AUC0-∞=AUC0-tlast+Ct/λz, where Ct is the last measurable concentration and λz is the terminal elimination rate constant. The ln-transformed AUC0-tlast, AUC0-∞ and Cmax were primary pharmacokinetic parameters. In addition, tmax, λz, half-life (t1/2), and %AUC extrapolation were reported as secondary parameters.
The statistical analysis was carried out using PROC GLM of SAS® Software Version 9.4 (SAS Institute Inc., USA). Analysis of Variance (ANOVA) was performed for ln-transformed AUC0-tlast, AUC0-∞ and Cmax. ANOVA model included sequence, formulation and period as fixed effects, and subject (sequence) as a random effect. Sequence effect was tested using subject (sequence) as an error term. The 90% Confidence Intervals (CIs) for the ratio of the geometric least squares mean (test/reference) of ln-transformed AUC0-tlast, AUC0-∞ and Cmax were calculated and bioequivalence between two formulations was to be concluded if the 90% CIs were within the acceptable range of 80.00%-125.00%. Wilcoxon signed rank test was performed to compare tmax of the test and reference products. All statistical calculations were executed at a significance level of 5% (α=0.05).
Results
Demographic characteristics of study subjects
A total of 50 subjects (25 males and 25 females) were enrolled this study. The mean ± SD of age, height, weight and BMI of enrolled subjects were 32.66 ± 7.68 years, 1.65 ± 0.08 m, 63.60 ± 9.87 kg and 23.18 ± 2.83 kg/m2, respectively. Three subjects dropped out from the study before dosing in period-II whereas another subject dropped out after blood sample collection at 24.00 hours post- dose due to personal reasons. Therefore, 47 subjects were dosed in period-II, but 46 subjects completed the study. The data from 46 subjects were used for statistical comparison.
Study sample analysis and incurred sample reanalysis
A total of 2,616 collected samples including samples from drop-out subjects were successfully analyzed using the validated LC-MS/MS method. The samples from the same subject were analyzed in the same analytical run. There were 6 samples accounted for 0.2% of total samples were reanalyzed. The correlation coefficient calculated from 8 calibration standards was more than 0.99 for all analytical runs. The inter-run accuracy of 6-level quality control samples ranged from 98.5% to 100.7% of their nominal concentrations. The Coefficient of Variation (CV) of quality control samples ranged from 1.9% to 4.6%. The ISR was carried out in two separate analytical runs for 194 selected samples. The difference between original and ISR concentrations of 193 incurred samples was less than 20% accounted for 99.5% of selected samples. The ISR results complied with the requirements as per EMA guideline on the validation of bioanalytical methods [16]. The reanalysis using incurred samples confirmed the reproducibility and reliability of the concentration data used for pharmacokinetic analysis.
Pharmacokinetic and statistical analysis
The data of primary pharmacokinetic parameters (AUC0-tlast, AUC0-∞ and Cmax) and secondary pharmacokinetic parameters (tmax, λz, t1/2, Extrapolated AUC (%)) of test and reference product from forty-six subject who completed the study are represented in Table 1. After oral administration of both products under fasting conditions, the mean AUC0-tlast was 190.092 ng.hr/mL and 188.473 ng.hr/mL for the test and reference formulations, respectively. The mean Cmax around 24 ng/mL was achieved at the median tmax of 3.3 hours for both formulations. The t1/2 and extrapolated AUC (%) values of the test product were slightly higher than reference product. However, the pharmacokinetic parameters of rosuvastatin were comparable between test and reference product. The mean plasma concentration-time profiles of rosuvastatin for the test and reference products are illustrated in Figure 1.
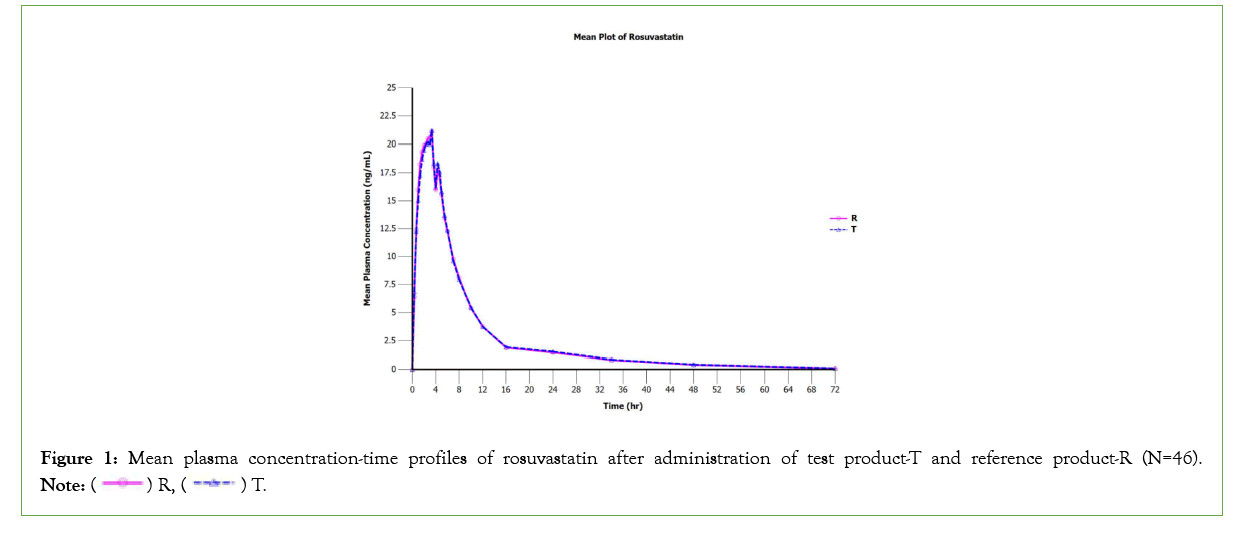
Figure 1: Mean plasma concentration-time profiles of rosuvastatin after administration of test product-T and reference product-R (N=46). 
| Parameters (Units) | Mean ± SD (untransformed data, N=46) | |
|---|---|---|
| Test product-T | Reference product-R | |
| AUC0-tlast (ng.hr/mL) | 190.092 ± 99.833 | 188.473 ± 92.927 |
| AUC0-∞ (ng.hr/mL) | 197.315 ± 101.422 | 193.672 ± 93.499 |
| Cmax (ng/mL) | 23.595 ± 14.479 | 23.985 ± 15.205 |
| tmax (hr, in median (min, max)) | 3.33 (1, 6) | 3.33 (1.33, 5) |
| λz (1/hr) | 0.059 ± 0.023 | 0.059 ± 0.022 |
| t½ (hr) | 14.192 ± 7.375 | 13.064 ± 4.292 |
| Extrapolated AUC (%) | 4.078 ± 4.932 | 3.092 ± 2.485 |
Table 1: Pharmacokinetic parameters for test and reference products of rosuvastatin.
The primary pharmacokinetic parameters data obtained from 46 subjects who completed the entire study were used for statistical analysis and the results are represented in Table 2. The ANOVA indicated no significant effects of formulation, sequence and period on ln-transformed primary pharmacokinetic parameters. The 90% CIs for the ratio of the geometric least squares mean (test/ reference) of ln-transformed AUC0-tlast, AUC0-∞ and Cmax were within the bioequivalence criteria of 80.00%-125.00%. No significant difference in median tmax between the two products as indicated by Wilcoxon signed rank test (p-value=0.4137).
| Parameters | Ratio (90% CI) | Power (%) | Intra-subject CV (%) | ANOVA (p-value) | ||
|---|---|---|---|---|---|---|
| Formulation | Sequence | Period | ||||
| ln AUC0-tlast | 97.9 (91.81-104.50) | 100 | 18.9 | 0.5938 | 0.548 | 0.2467 |
| ln AUC0-∞ | 99.1 (93.26-105.29) | 100 | 17.6 | 0.8017 | 0.5094 | 0.353 |
| ln Cmax | 97.6 (89.88-105.96) | 99.7 | 24.1 | 0.621 | 0.4254 | 0.0676 |
Table 2: Results of statistical comparison of primary parameters of rosuvastatin between test and reference products.
Tolerability
The incidence of adverse events was monitored for tolerability assessment. Three adverse events were reported in this study which are increased alanine aminotransferase (ALT), increased blood glucose and decreased hemoglobin and hematocrit, and their incidence is listed in Table 3. There was no serious adverse event reported in this study. All of adverse events were mild in intensity and unlikely to be clinically relevant.
| Adverse events | Incident report (N) | |
|---|---|---|
| Test product | Reference product | |
| Increased ALT | 1 | 0 |
| Increased blood glucose | 1 | 0 |
| Decreased hemoglobin and hematocrit | 0 | 3 |
| Total | 2 | 3 |
Table 3: List of adverse events.
Discussion
Rosuvastatin is rapidly absorbed, reaching peak plasma concentration within 3 to 5 hour after dosing. This bioequivalence study was carried out under fasting conditions since the extent of absorption was similar when given with or without food [10]. In general, conduct of a bioequivalence study under fasting conditions is considered to be the most sensitive condition to detect a difference of the formulation [15]. The concentration-time profiles and the pharmacokinetic parameters were comparable between the test and reference products. Fourteen-days of washout period was sufficient for complete drug elimination since no concentration was detected in any pre-dose samples of period II. When comparing with the previously reported data, this study demonstrated similar values of AUC0-∞, Cmax and tmax for the reference product CRESTOR® 40 mg tablets administered in healthy Colombian volunteers. The mean elimination rate constant observed in 30 Colombian subjects was almost 2-fold higher than that observed in Thai healthy subjects [17]. In contrast, mean elimination rate constant reported in 28 Chinese subjects was comparable to the value report in Thai subjects for the study conducted in fasting states [18]. Considering dose-normalized AUC0-∞ and Cmax in Indonesian subjects, those parameters were significantly higher than the observed values in Thai population. However, the tmax was achieved within the similar range between these two populations [19]. The genetic polymorphisms of drug metabolism could potentially contribute to the variability among different ethnicity [20].
The maximum intra-subject variability for primary pharmacokinetic parameter Cmax of rosuvastatin was found to be around 28.5%. The intra-subject variability for Cmax observed in this study was similar to the reported value, and it is thus not considered as a highly variable drug [13]. The sample size computation yield that 40 subjects would be sufficient to establish bioequivalence with adequate power. Therefore, the evaluable data from 46 subjects who completed the study contributed to the demonstration of bioequivalence with the power nearly 100.0%. The results of bioequivalence was concluded by the 90% CIs for the ratio of the geometric least squares mean of ln-transformed AUC0-tlast, AUC0-∞ and Cmax which were within the acceptance criteria of 80.00%-125.00%. From ANOVA results, there were no significant effects of formulation, period and sequence on the ln-transformed primary pharmacokinetic parameters (p>0.05). Moreover, Wilcoxon signed rank test showed no significant difference between two formulations in median of tmax. The results of this study showed that two rosuvastatin formulations were bioequivalent in terms of the rate and extent of absorption.
Based on the product monograph of the reference product, CRESTOR®, the most frequently reported adverse events were arthralgia, upper abdominal pain and elevated ALT [10]. The increased ALT, increased blood glucose and hematologic changes observed in this study were mild in intensity. Moreover, they were not considered to be clinically significant and the study subjects recovered without medicinal treatment. However, the tolerability upon continuous use should be further investigated.
Conclusion
The statistical comparison of primary pharmacokinetic parameters (AUC0-tlast, AUC0-∞ and Cmax) between the test product, ROZACOR® and reference product, CRESTOR® of rosuvastatin 20 mg tablets indicated that these products were bioequivalent. The 90% CIs of the geometric least squares mean ratio between the formulations for ln-transformed primary pharmacokinetic parameters were within the acceptance range of 80.00%-125.00%. There were no serious adverse events reported in this study, and both test and reference formulations were well tolerated by the study subjects. The usage of ROZACOR® as a generic alternative to CRESTOR® was supported by the bioequivalence study in healthy Thai volunteers under fasting conditions
Acknowledgement
This study was supported by the Government Pharmaceutical Organization (GPO), Thailand.
References
- Hill MF, Bordoni B. Hyperlipidemia. In: StatPearls. Treasure Island (FL). StatPearls Publishing. 2022.
[Google Scholar], [PubMed]
- Alloubani A, Nimer R, Samara R. Relationship between Hyperlipidemia, Cardiovascular Disease and Stroke: A Systematic Review. Curr Cardiol Rev. 2021;17(6):e051121189015.
[Crossref], [Google Scholar], [PubMed]
- Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40(1):195-211.
[Crossref], [Google Scholar], [PubMed]
- Kennewell PD. 1.03 - Major Drug Introductions. In: Taylor JB, Triggle DJ, editors. Comprehensive Medicinal Chemistry II. Oxford: Elsevier; 2007:97-249.
[Crossref]
- Quirk J, Thornton M, Kirkpatrick P. Rosuvastatin calcium. Nat Rev Drug Discov. 2003;2(10):769-770.
[Google Scholar], [PubMed]
- Maji D, Shaikh S, Solanki D, Gaurav K. Safety of statins. Indian J Endocrinol Metab. 2013;17(4):636-646.
- Vardanyan R, Hruby V. Chapter 20 - Hypolipidemic and Antihyperlipidemic Drugs. In: Vardanyan R, Hruby V, editors. Synthesis of Best-Seller Drugs. Boston: Academic Press; 2016:285-315.
[Crossref], [Google Scholar]
- White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42(9):963-970.
[Google Scholar], [PubMed]
- Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19(1):117-125.
[Crossref], [Google Scholar], [PubMed]
- AstraZeneca Canada Inc. Product Monograph Crestor (Rosuvastatin Calcium) Tablets 5, 10, 20 and 40 mg. Canada: AstraZeneca; 2022.
- Warwick MJ, Dane AL, Raza A, Scheneck DW. Single and multiple dose pharmacokinetics and safety of the new HMG CoA reductase inhibitor ZD4522. Atherosclerosis. 2000;151(1):39.
[Crossref], [Google Scholar]
- InterASIA Collaborative Group. Cardiovascular risk factor levels in urban and rural Thailand--The International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). Eur J Cardiovasc Prev Rehabil. 2003;10(4):249-257.
[Google Scholar], [PubMed]
- Thota S, Tippabhotla SK, Khan SM, Nakkawar M, Venkateswarlu V. Two-way crossover, bioequivalence study of rosuvastatin tablets 5 mg in healthy, adult, Asian-Indian male volunteers under fasting condition. Int J Pharm Pharm Sci. 2013;5:289-293.
- Zhang P. A simple formula for sample size calculation in equivalence studies. J Biopharm Stat 2003;13(3):529-538.
[Crossref], [Google Scholar], [PubMed]
- European Medicines Agency. Guideline on the Investigation of Bioequivalence. Committee for Medicinal Products for Human Use (CHMP), London. 2010.
- European Medicines Agency. Guideline on bioanalytical method validation. Committee for Medicinal Products for Human Use (CHMP), London. 2011.
- Vargas M, Bustamante C, Ea V. Bioequivalence Study of Two Formulations Containing Rosuvastatin 40Mg Tablets in Healthy Colombians. J Bioequiv Availab. 2015;7(5):229-232.
[Crossref], [Google Scholar]
- Zhu KW, Wang GM, Li CY, Liu JY, Huang JY, Wu JR, et al. Pharmacokinetics and Bioequivalence of Two Formulations of Rosuvastatin Following Single-dose Administration in Healthy Chinese Subjects Under Fasted and Fed Conditions. Clin Pharmacol Drug Dev. 2022;11(8):987-996.
[Crossref], [Google Scholar], [PubMed]
- Harahap Y, Prasaja B, Azmi F, Lusthom W, Sinandang T, Felicia V, et al. Bioequivalence study of two rosuvastatin tablet formulations in healthy Indonesian subjects. Int J Clin Pharmacol Ther. 2016;54(3):212-216.
[Crossref], [Google Scholar], [PubMed]
- Kanukula R, Salam A, Rodgers A, Kamel B. Pharmacokinetics of Rosuvastatin: A Systematic Review of Randomised Controlled Trials in Healthy Adults. Clin Pharmacokinet. 2021;60(2):165-175.
[Crossref], [Google Scholar], [PubMed]
Citation: Vattanarongkup J, Seeduang C, Kunsa-ngiem S, Khaowroongrueng V, Saeaue L, Karachot B, et al. (2023) Bioequivalence Study of Rosuvastatin 20 mg Tablets in Healthy Thai Volunteers under Fasting Conditions. J Bioequiv Availab. 15:508.
Copyright: © 2023 Vattanarongkup J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

