PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 15, Issue 3
Bioequivalence and Pharmacokinetics of a Fixed-Dose Combination of Dolutegravir, Lamivudine and Tenofovir Disoproxil Fumarate in Healthy Thai Volunteers
Wiwat Supasena1, Ekawan Yoosakul1, Mathus Sawpitiporn1, Charinthon Seeduang1, Suchada Rakphung1, Anas Sunhem1, Mariam Duereh1, Jaturavit Vattanarongkup1, Vipada Khaowroongrueng1*, Lalinthip Saeaue1, Busarat Karachot1, Piengthong Narakorn, Porranee Puranajoti2 and Isariya Techatanawat12International Bio Service Co., Ltd., 888 Golden Jubilee Medical Center, Mahidol University, Nakhon Pathom, Thailand
Received: 28-Feb-2023, Manuscript No. JBB-23-20233; Editor assigned: 03-Mar-2023, Pre QC No. JBB-23-20233 (PQ); Reviewed: 17-Mar-2023, QC No. JBB-23-20233; Revised: 24-Mar-2023, Manuscript No. JBB-23-20233 (R); Published: 31-Mar-2023, DOI: 10.35248/0975-0851.23.15.518
Abstract
A regimen of dolutegravir, lamivudine and tenofovir disoproxil fumarate has been approved to treat Human Immunodeficiency Virus (HIV) infection. To enhance patient adherence and accessibility to combined antiretroviral therapy, a fixed-dose combination formulation for this regimen had been developed. A comparative randomized, single dose, two-way crossover, open-label study was conducted in 52 healthy Thai volunteers to evaluate the bioequivalence and pharmacokinetics of the fixed-dose combination compared with those of separate tablets. Blood samples were collected through 72 hours post-dose. The concentrations of dolutegravir, lamivudine and tenofovir, an active metabolite of tenofovir disoproxil fumarate in the processed plasma samples were determined using two validated LC-MS/MS methods. The primary pharmacokinetic parameters were the area under the plasma concentration-time curve (AUC0-tlast) and the maximum concentration of drug in plasma (Cmax). The results showed that the fixed-dose combination was bioequivalent to the reference products in terms of rate and extent of absorption of each drug as indicated by the 90% confidence intervals of the geometric least squares mean ratios (test/reference) for ln-transformed AUC0-tlast and Cmax, which were within the acceptance range of 80.00%-125.00%. Both products were well tolerated by the study subjects. There were no serious adverse events reported in this study. The results support the use of fixed-dose combination product as an alternative product of three separate tablets.
Keywords
Dolutegravir; Lamivudine; Tenofovir disoproxil fumarate; Tenofovir; Bioequivalence; Pharmacokinetics; Fixed-dose combination
Introduction
The infection of Human Immunodeficiency Virus (HIV) leads to massive depletion of CD4+ T cells which is associated with high viral replication. Patients may experience flu-like symptoms with other symptoms such as anorexia and diarrhea during the early- phase infection. Without antiretroviral therapy, HIV induces immune dysfunction that persists throughout the chronic phase, thereby making infected patients susceptible to the opportunistic diseases leading to death among patients [1]. Effective antiretroviral therapy has been invented to reduce morbidity and mortality in patients [2]. A concept of combination therapy involving the use of antiretroviral drugs from different classes such as Nucleoside Reverse Transcriptase Inhibitors (NRTIs), Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs), Protease Inhibitors (PIs), Integrase Strand Transfer Inhibitors (INSTIs), and Chemokine (C-C Motif) Receptor 5 (CCR5) antagonists has been introduced to reduce the occurrence of drug resistance and toxicity [3,4].
According to the recommendations of World Health Organization (WHO) and the U.S. Department of Health and Human Services, the combination of an INSTI and two NRTIs, such as Dolutegravir (DTG), Lamivudine (3TC) and Tenofovir Disoproxil Fumarate (TDF), is recommended as an alternative first-line regimen for adults [5,6]. DTG exhibits antiviral activity via blocking integrase enzyme which HIV uses to integrate viral DNA into host CD4+ T cell. It has demonstrated the efficacy in both antiretroviral-naïve and experienced patients. A combination use of DTG with other two NRTIs becomes manageable due to minimal drug-drug interaction [7]. 3TC is a deoxycytidine analogue whereas TDF is a prodrug of Tenofovir (TFV) which is deoxyadenosine analogue. Due to absence of hydroxy group on both molecules, reverse transcriptase DNA synthesis is terminated [8]. Similarly, 3TC and TDF have limited potential for drug-drug interactions via Cytochrome P (CYP) 450 pathway [9,10].
Given that patients are required to take antiretrovirals routinely for life, adherence to a separate tablet regimen is costly and unachievable for some patients which could result in increased risk of drug resistance development. Therefore, the Government Pharmaceutical Organization (GPO), Thailand had developed a fixed-dose combination of DTG, 3TC, and TDF 50/300/300 mg as an alternative product with a reduced cost to improve drug accessibility and patients’ quality of life. To ensure product quality and safety, a bioequivalence study between the fixed-dose combination product and the reference product as separate tablets was conducted in healthy Thai volunteers.
Materials and Methods
Study product
The test product of DTG, 3TC, and TDF 50/300/300 mg fixeddose combination tablets (DALAVIR) was manufactured by GPO, Thailand bearing lot No. S655030. The reference products were TIVICAY (DTG 50 mg tablets) manufactured by GlaxoSmithKline, Belgium for ViiV healthcare, bearing lot No. 774U, EPIVIR (3TC 300 mg tablets) manufactured by GlaxoSmithKline, Canada for ViiV healthcare, bearing lot No. 2P5N and VIREAD (TDF 300 mg tablets) manufactured by Gilead Sciences, Inc, Canada, bearing lot No. 027985.
Study subjects
The number of subjects was estimated based on the maximum intra-subject variability for maximum concentration (Cmax) of DTG which was approximately 25.5% (in-house data). The calculation was done by assuming the geometric least squares mean ratio (test/ reference) of ln-transformed primary pharmacokinetic parameters at 95%, significant level at 5% and power greater than 90%. Thirtyeight subjects were required for the study; however, the additional 35% of subjects were considered for compensation of any dropout and withdrawal resulting a total of 52 subjects.
All subjects were healthy males and non-pregnant females at the age of 18-55 years with a Body Mass Index (BMI) 18.0-30.0 kg/m2 were screened through medical history, physical examination and laboratory examinations on blood chemistry, hematology, hepatitis B antigen, anti-HIV, RNA of SARS-CoV-2, drugs abuse and alcohol consumption. All subjects were monitored for adverse events and the severity was determined as mild, moderate, or severe during the study by the physician. The written informed consent was given by the study subjects before study participation at International Bio Service Co., Ltd., Golden Jubilee Medical Center, Mahidol University, Thailand.
Study design
An open label, randomized, two-treatment, two-period, twosequence, single oral dose, crossover study was conducted to evaluate bioequivalence between the test product, DALAVIR (DTG, 3TC, and TDF 50/300/300 mg tablets) and the reference products TIVICAY (DTG 50 mg tablets), EPIVIR (3TC 300 mg tablets) and VIREAD (TDF 300 mg tablets) in healthy, adult subjects under fasting conditions. The study protocol was approved by Institute for the Development of Human Research Protections (IHRP), Department of Medical Sciences, Ministry of Public Health, Thailand. The study was conducted in accordance with the Declaration of Helsinki and its amendments, the International Conference on Harmonization (ICH) Guideline for Good Clinical Practice, European Medicines Agency (EMA) guideline on the investigation of bioequivalence, EMA guideline on clinical development of fixed combination medicinal products, and U.S. Food and Drug Administration (FDA) guidance for industry on bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an Abbreviated New Drug Application (ANDA) [11-15].
The randomization schedule was generated for determining the receiving order of the test and reference products for each subject using SAS® version 9.4 (SAS Institute Inc., USA). The subjects received either a tablet of test product or three tablets of reference product orally after an overnight fasting in period I. Then, they received an alternate treatment after a 14-day washout period. Taking medications other than the investigational medicinal products were not allowed for 14 days before the study till completion of the study
Blood sampling
Blood samples were collected from each subject in each period at pre-dose (0 hour) and 0.17, 0.33, 0.5, 0.67, 0.83, 1, 1.25, 1.5, 1.75, 2, 2.33, 2.67, 3, 3.5, 4, 5, 6, 8, 12, 16, 24, 34, 48 and 72 hours post-dose. An indwelling intravenous cannula was placed in a forearm vein of each subject for serial blood sampling. The samples were then transferred into vacutainers containing dipotassium ethylenediaminetetraacetate (K2 EDTA) as the anticoagulant and placed upright in a wet ice water bath until centrifugation. The samples were centrifuged at 3000 ± 100 rcf for 5 minutes at below 10°C to separate plasma. All separated plasma samples were transferred to pre-labeled polypropylene tubes and stored at -50°C or colder until sample analysis at Bioequivalence Study Group, Research and Development Institute, GPO, Thailand.
Study sample analysis
The plasma samples were analysed using two separate validated LC-MS/MS methods. One was for analysis of DTG individually and the other one was for simultaneous analysis of 3TC and TFV. The employed LC-MS/MS system was NexeraTM Ultra-Performance Liquid Chromatography (UPLC) system (Shimadzu Corporation, Kyoto, Japan) coupled with TSQ Quantum Ultra mass spectrometer (Thermo Fisher scientific, Massachusetts, USA). Data acquisitions and processing were accomplished using XcaliburTM 4.0.27.42 and LCquanTM 3.0.26.0 (Thermo Fisher scientific, Massachusetts, USA).
For an analysis of DTG, plasma concentration of DTG was measured against a calibration curve ranging from 20.626 to 5882.616 ng/mL. Dolutegravir-d5 was used as an internal standard. The samples were processed by protein precipitation technique using methanol. The supernatant was injected into ACE 5 C18 100 × 4.6 mm analytical column (Advanced Chromatography Technologies Ltd, Aberdeen, Scotland), of which the temperature was maintained at 50°C. An isocratic mobile phase consisted of 0.1% formic acid solution (v/v) and methanol at a ratio of 40:60 (v/v). Mass spectrometric analysis was conducted in multiple reaction monitoring (MRM) using positive ionization mode at mass-to-charge ratio (m/z) transitions of 420.155 → 277.090 and 425.172 → 277.090 for DTG and dolutegravir-d5, respectively.
For a simultaneous analysis of TFV and 3TC, the sample preparation was carried out using Oasis MCX 1 cc/30 mg Solid-Phase Extraction (SPE) cartridges, which were conditioned using methanol followed by water. They were then washed using 0.1 N hydrochloric acid solution and methanol after sample loading. The analytes were eluted by 1.25% ammonia in methanol (w/v). The eluent was evaporated at 40°C and subsequently reconstituted using 2 mM ammonium acetate (pH 7.0):methanol (50:50, v/v). Tenofovir-d7 and lamivudine-13C-15N2 were used as internal standards for TFV and 3TC, respectively. The chromatographic separation was achieved using SeQuant® ZIC®-HILIC 3.5 μm, 200Å 50 × 2.1 mm (Merck KGaA, Darmstadt, Germany) as an analytical column and 2 mM ammonium acetate (pH 7.0):methanol (30:70, v/v) as a mobile phase. Plasma concentration was determined in the calibration ranges of 5.095 to 799.900 ng/mL for TFV and 15.325 to 5527.474 ng/mL for 3TC. The m/z transitions in positive ionization mode of TFV, tenofovir-d7, 3TC and lamivudine-13C-15N2 were set at 288.060→176.040, 295.078→183.120, 230.083→112.090 and 233.086→115.100, respectively.
Pharmacokinetic and statistical analysis
The pharmacokinetic analysis was done for DTG, 3TC and TFV individually. All parameters were derived from plasma concentration vs. time profiles for each analyte. Phoenix WinNonlin Software Version 6.4 (Pharsight Corporation, USA) was used for noncompartmental analysis. The primary pharmacokinetic parameters consisting of maximum concentration (Cmax) and the area under the plasma concentration-time curve from time zero to the last observed time point (AUC0-tlast) were used for statistical analysis to determine bioequivalence between the test and reference. The secondary pharmacokinetic parameters e.g., the area under the plasma concentration-time curve from time zero to infinity (AUC0-∞), time to maximum concentration (tmax), elimination rate constant (λZ), half-life (t1/2), and %AUC extrapolation were also computed and reported.
The statistical analysis was performed using PROC GLM of SAS® Software Version 9.4 (SAS Institute Inc., USA). Analysis of Variance (ANOVA) was performed for ln-transformed AUC0-tlast, and Cmax of DTG, 3TC and TFV. The ANOVA model included sequence, formulation and period as fixed effects, and subject (sequence) as a random effect. Sequence effect was tested using subject (sequence) as an error term. The geometric least squares mean ratios (test/ reference) of ln-transformed AUC0-tlast and Cmax and related 90% Confidence Intervals (CIs) were calculated. Additionally, the difference in tmax between test and reference products was assessed using Wilcoxon signed rank test. The bioequivalence between two formulations was to be concluded if the 90% CIs were within the acceptable range of 80.00%-125.00%.
Results
Demographic data
In this study, 55 subjects were enrolled; however, only 52 subjects were dosed in period I as per the sample size calculation. After dosing in period I, one subject was withdrawn due to taking the reference products with the water less than the pre-defined volume in the protocol. Therefore, fifty-one subjects had their blood samples collected till the end of period. In period II, five subjects were withdrawn due to positive RT-PCR test for SARS-CoV-2 and one subject was withdrawn due to positive urine screening test for drug abuse. Consequently, forty-five subjects completed the study and their data were used for bioequivalence evaluation. The demographic data of the subjects who completed both study periods are summarized in Table 1.
| Variable | Mean (SD) (n=45) |
|---|---|
| Age (years) | 36.60 (9.36) |
| Weight (kg) | 65.80 (10.59) |
| Height (m) | 1.65 (0.08) |
| BMI (kg/m2) | 24.08 (2.86) |
Table 1: Baseline demographic of subjects who completed the study.
Pharmacokinetic and statistical analysis
The mean concentration-time profiles of forty-five subjects following the test and reference products administration are illustrated in Figure 1. The primary and secondary pharmacokinetic parameters are presented in Table 2. The 90% Confidence Intervals (CIs) for the ratios of geometric least squares mean of ln-transformed AUC0-tlast and Cmax of all analytes were within the acceptance range of 80.00%-125.00% (Table 3). ANOVA results reveal that there was a significant formulation effect on both lntransformed AUC0-tlast and Cmax of DTG and ln-transformed Cmax of 3TC whereas there was a significant sequence effect on lntransformed Cmax of TFV (p<0.05). Wilcoxon signed rank test showed no significant difference between two formulations in median tmax for DTG. However, median tmax of 3TC and TFV was found to be significantly different between two formulations.
| Variable | Dolutegravir (n=45) | Lamivudine (n=45) | Tenofovir (n=45) | |||
|---|---|---|---|---|---|---|
| Test | Reference | Test | Reference | Test | Reference | |
| AUC0-tlast (ng.h/mL) | 66861.459(14671.523) | 62075.382(15863.550) | 12739.612(3193.228) | 12518.236(3277.880) | 2870.952(640.362) | 2881.213(725.516) |
| AUC0-8(ng.h/mL) | 70708.451(16287.630) | 66082.412(17576.092) | 13119.378(3216.149) | 12844.128(3356.992) | 3105.153(641.720) | 3088.749(730.648) |
| Cmax (ng/mL) | 2961.600(663.676) | 2803.824(794.128) | 2165.658(582.562) | 2390.102(616.509) | 336.949(108.468) | 373.684(141.252) |
| tmax (h)* | 3.25(0.5-8) | 2.89(0.5-6) | 2.22(0.83- 4) | 1.66(0.67-4) | 1.25(0.67-5) | 0.90(0.5-2.67) |
| ?z (1/h) | 0.044(0.0085Â) | 0.043(0.0086) | 0.102(0.0608) | 0.112(0.0710) | 0.040(0.0070) | 0.041(0.0067) |
| t1/2 (h) | 16.218(3.426) | 16.845(3.683) | 10.978(9.334) | 10.328(8.424) | 17.680(3.194) | 17.383(2.840) |
| Extrapolated AUC (%) | 5.151(3.283) | 5.730(3.615) | 2.941(2.910) | 2.516(1.994) | 7.830(3.378) | 7.061(2.689) |
| Note: *tmax values are represented in median (Min-Max) value. | ||||||
Table 2: Pharmacokinetic parameters of Dolutegravir, Lamivudine and Tenofovir for test (DALAVIR) and reference (TIVICAY+EPIVIR+VIREAD). The data are mean (SD).
| Parameters | Dolutegravir (n=45) | Lamivudine (n=45) | Tenofovir (n=45) | |||
|---|---|---|---|---|---|---|
| Ratio of Test/Reference | 90% CI | Ratio of Test/Reference | 90% CI | Ratio of Test/Reference | 90% CI | |
| ln AUC0-tlast | 108.70% | 103.67%-113.88% | 102.10% | 98.67%-105.64% | 100.30% | 96.44%-104.22% |
| ln Cmax | 107.20% | 101.72%-112.99% | 90.50% | 86.88%-94.27% | 91.90% | 84.76%-99.71% |
Table 3: Statistical comparison of primary parameters of Dolutegravir, Lamivudine and Tenofovir between test (DALAVIR) and reference products (TIVICAY+EPIVIR+VIREAD).
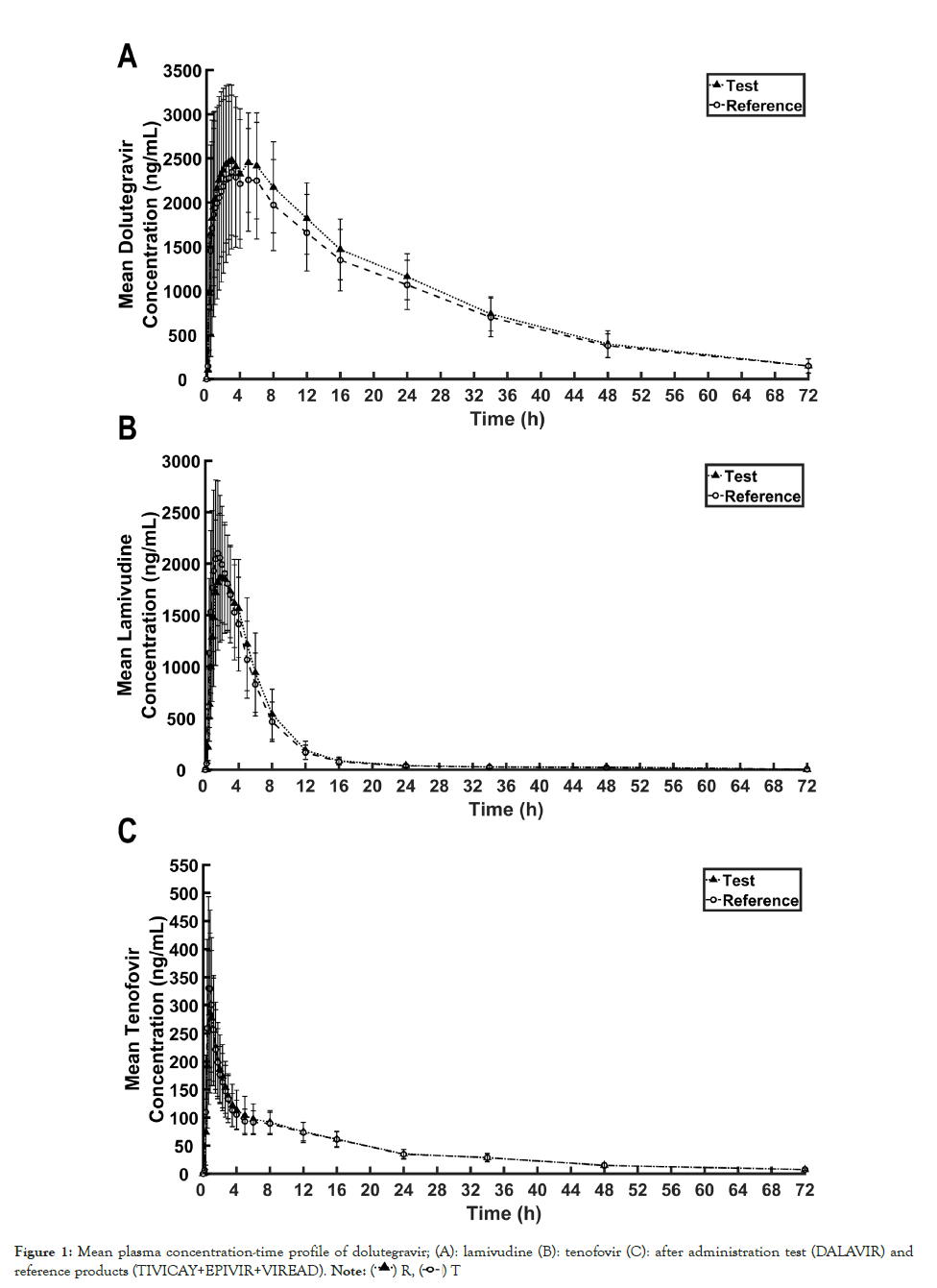
Figure 1: Mean plasma concentration-time profile of dolutegravir; (A): lamivudine (B): tenofovir (C): after administration test (DALAVIR) and reference products (TIVICAY+EPIVIR+VIREAD). Note
Tolerability
Thirty-three adverse events were reported in 28 subjects. All adverse events were mild in the intensity and no further follow-up was required. The causality was assessed to be possible related to the treatment for 26 adverse events, unrelated for 5 adverse events (positive RT-PCR test for SARS-CoV-2) and unlikely for 2 adverse events (loose stool and epigastric pain). Decreased hemoglobin and hematocrit were mostly observed in this study, which were reported in six subjects after administration of test product and in four subjects after administration of reference product. The incidences of all adverse events are summarized in Table 4.
| Adverse event | Number of adverse events | |
|---|---|---|
| Test | Reference | |
| Loose stool | 1 | 0 |
| Epigastric pain | 0 | 1 |
| Increased alkaline phosphatase | 1 | 0 |
| Increased creatine phosphokinase | 1 | 1 |
| Increased creatinine | 0 | 1 |
| Decreased hemoglobin and hematocrit | 6 | 4 |
| Increased monocyte | 0 | 1 |
| Decreased/increased lymphocyte | 2 | 0 |
| Decreased/increased Neutrophil (segmented) | 2 | 0 |
| Increased platelet count | 1 | 1 |
| Decreased white blood cell count | 0 | 3 |
| Headache | 2 | 0 |
| COVID-19 Infection | 2 | 3 |
| Total adverse event | 18 | 15 |
Table 4: List of adverse events.
Discussion
The bioequivalence study was conducted to compare the pharmacokinetic parameters between fixed-dose combination of DTG, 3TC and TDF of the test product and three separate tablets of the reference product at equivalent doses. It is noteworthy that the reference products employed to this bioequivalence study were selected from the approved medicinal products in ICH countries. The study design was established in accordance with EMA guideline on the investigation of bioequivalence, EMA Guideline on clinical development of fixed combination medicinal products, and U.S. Food and Drug Administration (FDA) guidance for industry on bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA [13-15].
According to WHO notes on the Design of Bioequivalence Study: Lamivudine/Tenofovir/Dolutegravir, TFV plasma concentration was measured instead of TDF due to rapid conversion of TDF to TFV after oral absorption, and thus bioequivalence of TDF was demonstrated using pharmacokinetics of TFV [16]. The pharmacokinetic parameters of the test and reference product were comparable in this study suggesting similar rate and extent of absorption between two treatments. The AUC0-∞ of DTG, 3TC and TFV were reliably estimated since the mean %AUC extrapolations of DTG, 3TC and TFV, which were less than 6%, 3%, and 8%, respectively suggesting that the sampling time points were appropriately designed and the AUC0-tlast covered at least 80% of AUC0-∞ [11]. In addition, the pharmacokinetics of DTG, 3TC and TFV of Thai population (Table 2) did not significantly differ from that observed in other studies [17-21]. Following administration of DTG, 3TC and TDF at 50, 300 and 300 mg doses, the Cmax of DTG, 3TC and TFV at 50, 300 and 300 mg doses was around 2400-3600 ng/mL, 2100-3300 ng/mL and 240- 330 ng/mL, respectively. The AUC0-∞ of DTG, 3TC and TFV was around 45000-65000 ng.h/mL, 11000-13500 ng.h/mL and 1900- 2600 ng.h/mL, respectively. Even though the results of Wilcoxon signed-rank test showed the significant difference in median tmax of 3TC and TFV between the test and reference products, neither rapid onset of action nor time-dependent adverse effect has been claimed for 3TC and TFV. Therefore, the insignificant difference in median tmax is not necessarily required for the conclusion on bioequivalence in this study.
Considering the results of ANOVA, the sequence effect was observed on Cmax of TFV, but this effect was not observed for DTG and 3TC. Given that only eligible subjects were enrolled in both study periods and they were randomized into Test-Reference (TR) and Reference-Test (RT) groups evenly, the procedure in both study periods were standardized strictly following the approved protocol. Besides, the maximum half-live of TFV was 18 hours, thus 7-day washout period was adequate as evidenced by no drug concentration in any pre-dose samples collected in period II. Therefore, the confounding with the unequal residual effect and with the formulation-by-period interaction can be ruled out. Although the sequence effect existed, it did not affect the results of bioequivalence as the ANOVA adjusted the product effect for the sequence effect [22]. Significant formulation effect was also detected for ln-transformed AUC0-tlast and Cmax of DTG and ln-transformed Cmax of 3TC. The bioequivalence was established using the evaluable data from 45 subjects who completed the study. According to the sample size calculation, 38 subjects was sufficient for bioequivalence evaluation at the power greater than 90%. The mean square error explaining the variation within the treatments of these parameters was much smaller than the variation between the treatment (data not shown) resulting in larger test statistic value (F-value). Additionally, the 90% CIs for the ratios of geometric least squares mean of ln-transformed AUC0-tlast and Cmax of DTG and ln-transformed Cmax of 3TC were within the acceptance criteria of 80.00%-125.00%. Therefore, a significant formulation effect did not interfere in the results of this study [23].
In terms of safety, the fixed-dose combination of DTG, 3TC and TDF did not cause any serious adverse events. Mild symptoms were observed after administration of both the test and reference products during the study, indicating that both products were well tolerated. However, the safety upon continuous use needs further investigation.
Conclusion
This study was conducted to demonstrate the equivalence in biopharmaceutics quality described by rate and extent of absorption between fixed-dose combination product and three-drug regimen of DTG, 3TC and TDF. Based on statistical inferences, the test and reference products were bioequivalent since the 90% CIs of the geometric least squares mean ratio between the formulations for ln-transformed primary pharmacokinetic parameters of DTG, 3TC and TDF (demonstrated using TFV) were within the acceptance range of 80.00%-125.00%. The results from this study support the use of fixed-dose combination product as an alternative product of three separate tablets.
Acknowledgement
This study was supported by the Government Pharmaceutical Organization (GPO), Thailand.
References
- Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol. 2011;6:223-248.
[Crossref] [Google Scholar] [PubMed]
- Lu DY, Wu HY, Yarla NS, Xu B, Ding J, Lu TR. HAART in HIV/AIDS Treatments: Future Trends. Infect Disord Drug Targets. 2018;18(1):15-22.
[Crossref] [Google Scholar] [PubMed]
- Moore RD, Bartlett JG. Dramatic decline in the HIV-1 RNA level over calendar time in a large urban HIV practice. Clin Infect Dis. 2011;53(6):600-604.
[Crossref] [Google Scholar] [PubMed]
- Domingo P, Vidal F. Combination antiretroviral therapy. Expert Opin Pharmacother. 2011;12(7):995-998.
- World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016.
- U.S. Department of Health & Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2022.
- Kandel CE, Walmsley SL. Dolutegravir - a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des Devel Ther. 2015;9:3547-3555.
[Crossref] [Google Scholar] [PubMed]
- Yoshida Y, Honma M, Kimura Y, Abe H. Structure, Synthesis and Inhibition Mechanism of Nucleoside Analogues as HIV-1 Reverse Transcriptase Inhibitors (NRTIs). Chem Med Chem. 2021;16(5):743-766.
[Crossref] [Google Scholar] [PubMed]
- Johnson MA, Moore KH, Yuen GJ, Bye A, Pakes GE. Clinical pharmacokinetics of lamivudine. Clin Pharmacokin. 1999;36(1):41-66.
[Crossref] [Google Scholar] [PubMed]
- Wassner C, Bradley N, and Lee Y. A Review and Clinical Understanding of Tenofovir: Tenofovir Disoproxil Fumarate versus Tenofovir Alafenamide. J Int Assoc Provid AIDS Care. 2020;19:2325958220919231.
[Crossref] [Google Scholar] [PubMed]
- World Medical Association Declaration of Helsinki (WMA). Ethical principles for medical research involving human subjects. 2013.
- European Medicines Agency (EMA), International Conference on Harmonisation - World Health Organization. Guideline for good clinical practice, ICH topic E6 (R2). 2016.
- European Medicines Agency (EMA). Guidance on the investigation of bioequivalence, CPMP/EWP/QWP/1401/98 Rev. 1. 2010.
- European Medicines Agency (EMA). Guideline on clinical development of fixed combination medicinal products. 2017.
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA. 2021.
- WHO Prequalification Team, World Health Organization. Notes on the Design of Bioequivalence Study: Lamivudine/Tenofovir/Dolutegravir. 2021.
- Dumitrescu TP, Peddiraju K, Fu C, Bakshi K, Yu S, Zhang Z, et al. Bioequivalence and Food Effect Assessment of 2 Fixed-Dose Combination Formulations of Dolutegravir and Lamivudine. Clin Pharmacol Drug Dev. 2020;9(2):189-202.
[Crossref] [Google Scholar] [PubMed]
- Mehta R, Wolstenholme A, Di Lullo K, Fu C, Joshi S, Crauwels H, et al. Bioequivalence of a Fixed-Dose Combination Tablet of the Complete Two-Drug Regimen of Dolutegravir and Rilpivirine for Treatment of HIV-1 Infection. Antimicrob Agents Chemother. 2018;62(9):748-818.
[Crossref] [Google Scholar] [PubMed]
- Abhyankar D, Shedage A, Gole M, Raut P. Pharmacokinetics of fixed-dose combination of tenofovir disoproxil fumarate, lamivudine, and efavirenz: results of a randomized, crossover, bioequivalence study. Int J STD AIDS. 2017;28(5):491-498.
[Crossref] [Google Scholar] [PubMed]
- Ethel CF, Gustavo AY, Emilia KH, Serebrinsky C, Gonzalez S, Elvira Z. Single-Dose Bioequivalence of a New Fixed-Dose Combination Tablet Containing Tenofovir Disoproxil Fumarate and Lamivudine. J Bioequiv Availab. 2011;3(10):236-243.
- Weller S, Chen S, Borland J, Savina P, Wynne B, Piscitelli SC. Bioequivalence of a dolutegravir, abacavir, and lamivudine fixed-dose combination tablet and the effect of food. J Acquir Immune Defic Syndr. 2014;66(4):393-398.
[Crossref] [Google Scholar] [PubMed]
- Zintzaras E. The existence of sequence effect in cross-over bioequivalence trials. Eur J Drug Metab Pharmacokinet. 2000;25(3-4):241-244.
[Crossref] [Google Scholar] [PubMed]
- Bapuji AT, Ravikiran HLV, Nagesh M, Syedba S, Ramaraju D, Reddy CRJ, et al. Bioequivalence Testing - Industry Perspective. Journal of Bioequiv Availab. 2010;02(5).
Citation: Supasena W, Yoosakul E, Sawpitiporn M, Seeduang C, Rakphung S, Sunhem A, et al. (2023) Bioequivalence and Pharmacokinetics of a Fixed-Dose Combination of Dolutegravir, Lamivudine and Tenofovir Disoproxil Fumarate in Healthy Thai Volunteers. J Bioequiv Availab. 15:518.
Copyright: © 2023 Supasena W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

