PMC/PubMed Indexed Articles
Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 0, Issue 0
Association of Low Serum Complement 3 with Worse Glomerular Filtration Rate in Patients with IgA Nephropathy Secondary to Psoriasis
Da-feng He1,2, Rong Wang1,2, Chun-lei Lu2, Shi-jun Li1, Chang-hua Liu2, Cai-hong Zeng1 and Zheng Tang1*2Nephrology Department, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, Jiangsu, China
Received: 28-Dec-2020 Published: 20-Jan-2021, DOI: 10.35248/2167-0269.21.s11.001
Abstract
Objective: Complement system is pivotal in the pathogenesis of psoriasis and IgA Nephropathy (IgAN). Few studies have examined the features of patients with IgAN secondary to Psoriasis (IgAN-Pso). The association of serum complement and renal function is unknown. This study was made to investigate the relationship between serum C3 and glomerular filtration rate in patients with IgAN-Pso.
Methods: In this retrospective cross-sectional study, eighty-five patients with IgAN without evidence of a secondary cause other than psoriasis were enrolled. Patients were divided into two groups: the serum ≥ 0.9 g/L group (n=56) and the serum <0.9 g/L group (n=29). We used CKD-EPI equation to estimate Glomerular Filtration Rate (eGFR) and Empower Stats software to assess the relationship study.
Results: Patients with low serum C3 showed lower eGFR level than those with normal serum C3 (88.7 ml/min/1.73 m2 [57.6-107] and 76.3 ml/min/1.73 m2 [51.2-102]). No statistically differences were found in the histological characteristics between the two groups. Univariate analysis showed a positive correlation between serum C3 and eGFR (β =-26.4, 95%CI: -3.4 to 56.1, P=0.086). After adjusting for confounding factors, the positive correlation between serum C3 and eGFR became statistically significant. The eGFR increased by 7.23 ml/min/1.73 m2 and 7.26 ml/min/1.73 m2 with each increase of 0.1 g/ L of serum C3 in the adjustment II and adjustment III model, respectively. The eGFR in patients with low C3 decreased by 27.8 ml/min/1.73 m2 and 17.2 ml/min/1.73 m2 compared with that in patients with normal C3 levels in the adjustment II and adjustment III model, respectively. Furthermore, curve fitting showed that serum C3 and eGFR had a non-linear positive correlation.
Conclusion: Decreased serum C3 was associated with poor renal function in patients with IgAN-Pso, suggesting that complement system could be participated in the pathogenesis of IgAN-Pso.
Keywords
Serum complement 3; Glomerular filtration rate; IgA nephropathy; Psoriasis; Renal biopsy
Introduction
Psoriasis is an immune-mediated, genetic disease that manifests in the skin, joints or both. The disease results in both physical and psychological burdens, and it is considered a major global health problem by the WHO [1-3]. Psoriasis has been reported as an independent risk factor for Chronic Kidney Disease (CKD) and glomerulonephritis in several studies [4-6]. The association between Immunoglobulin A Nephropathy (IgAN) and psoriasis has been sporadically reported in few studies [7,8]. Patients with moderate-tosevere psoriasis had an increased risk of IgAN [9]. IgAN under this condition was often considered to be secondary Immunoglobulin A Nephropathy (sIgAN), although there is no consistent definition of sIgAN in the literature [10].
Systemic complement activation was reported a prominent role in psoriasis, mainly via the classical pathway and the alternative pathway [11]. In animal models of psoriasis, decreased C3 could reduce skin disease and activation of IL-23/IL-17 Axis of adaptive immunity [12,13]. Complement was considered to be one of the initiating factors in the pathogenesis of psoriasis, linking the innate auto-inflammatory and adaptive auto-immune responses in early stage of psoriasis [11].
Complement activation plays a critical role in the pathogenesis and clinical expression of IgAN [14]. Decreased plasma C3 levels with increased C3 activation products (iC3b and C3d) were found in some IgAN patients. In addition, low serum C3 level (90 mg/dl) predicted a worse outcome [15]. Activation of C3 is a biomarker of renal injury activity in IgAN patients [14]. C3aR/C5aR deficiency mouse presented reduced proteinuria and attenuated histological injury, generated IgAN model by Sendai virus infection [16]. Inhibitors of C3 activation were proposed to be potential candidates for IgAN treatment by animal and in vitro studies [17,18]. APL-2 (a C3 inhibitor) is being evaluated in a phase 2 study as treatment for patients with IgAN, lupus nephritis, primary membranous nephropathy or C3 glomerulopathy (NCT03453619) [19].
However, the association of complement and renal function in IgAN secondary to psoriasis is unknown. The aim of this study was to investigate the relationship between serum C3 and Glomerular Filtration (GFR) in patients with IgAN-Pso.
Methods
Patients
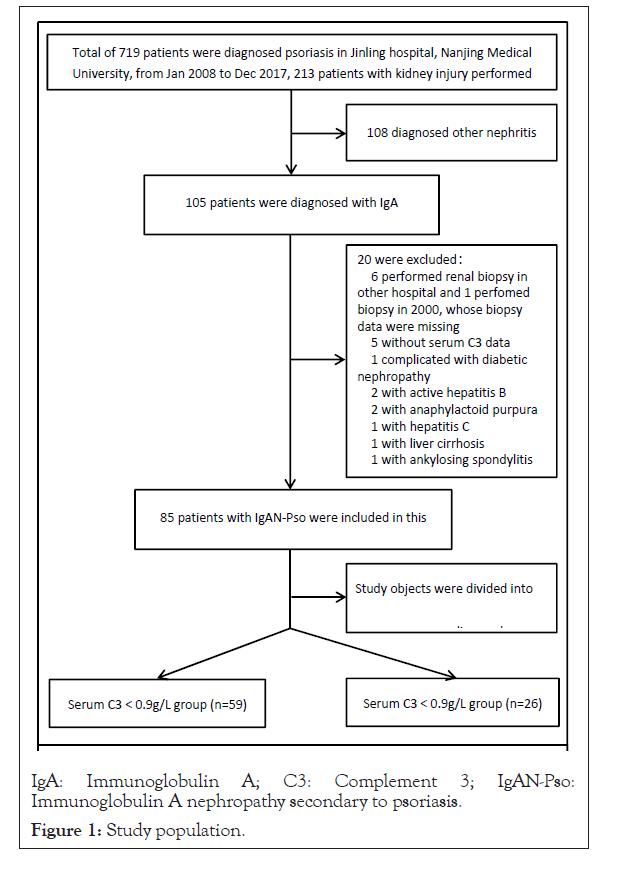
In this retrospective observational study, we reviewed the records of patients with psoriasis between 2008 and 2017 at the National Clinical Research Center of Kidney Diseases, Jinling hospital, Nanjing Medical University. A total of 213 patients underwent renal biopsies during the 10 years, 105 out of them were diagnosed with IgAN. 20 patients were excluded: 6 patients performed renal biopsy in other hospitals, 2 with active hepatitis B, 2 with Henoch- Schonlein purpura nephritis, 1 with hepatitis C, 1 with liver cirrhosis, 1 with diabetic nephropathy, 4 patients without serum complement 3(C3) data. 85 patients with IgAN without evidence of a secondary cause other than psoriasis were diagnosed IgAN-Pso, and were enrolled in the present study (detailed in Figure 1).
Figure 1: Study population.
In addition, one male patient with chronic hepatitis B was included in this study, because she was diagnosed with IgAN-Pso by a kidney specialist and a renal pathologist. Patients were divided into two groups: the serum ≥ 0.9 g/L group (n=56) and the serum <0.9 g/L group (n=29), according to the serum C3 levels.Diagnosis of psoriasis
The diagnosis of psoriasis was made by at least one dermatologist in our hospital or other tertiary care hospital [20]. We reviewed the records of patients with psoriasis to confirm the presence of typical psoriasis skin lesions, including red macules and papules with adherent silvery scales, the thin film characteristic, and evidence of dot haemorrhage [21].
Study factors
Demographic, clinical, and laboratory data at the time of renal biopsy and renal histopathology variables were gathered and compared between the two groups. The main pathological features observed in this study included glomerular sclerosis, Segmental glomerulosclerosis (S) lesion, Mesangial hypercellularity (M) lesion, Endocapillary hypercellularity (E) lesion, Crescents (C), Tubular atrophy/interstitial fibrosis (T) lesions, the presence of Glomerulus- Bowman’s Capsule Adhesion (GBCA) and capillary Necrosis (N). Small artery (especially the interlobular artery) lesions were also assessed. Immunofluorescence for Immunoglobulin G (IgG), Immunoglobulin A (IgA), Immunoglobulin M (IgM), C3, and complement 1q deposits were semi-quantitatively graded from 0 to 3 depending on the fluorescence intensity. Localization of deposits was observed by immunofluorescence and electron microscope [22].
Definitions of other terms
Psoriasis duration was defined as the time from the presence of typical rash to the time of renal biopsy. Gross haematuria was defined as >10,000/μl. Hypertension was diagnosed according to the standards advocated by the World Health Organization Expert Committee. Low serum C3 was identified as serum levels of C3<0.9 g/l. Kidney function was assessed by the estimated Glomerular Filtration Rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [23]. Renal dysfunction was identified as an eGFR <60 mL/min/1.73 m2.
The presence of E lesion was recorded as E1, the presence of S lesion or tuft adhesion was recorded as S-adhesion, and the presence of S lesion without tuft adhesion was recorded S-alone. T lesions were semi-quantitatively graded as T0 (0-24.9%), T1 (25%-50%) and T2 (>50%) according to the Oxford Classification1 [24,25]. C lesions were semi-quantitatively graded as C0 (no crescents), C1 (crescents in more than zero but less than one fourth of glomeruli), and C2 (crescents in one fourth or more of glomeruli) [26].
Statistical analysis
All the statistical analysis was performed using the Empower Stats and R software version 3.6.1. Data were expressed as medians (Interquartile Ranges, [IQRs]) for continuous variables and numbers of positive cases (percentages) for non-continuous variables. Covariate screening was analysed using computer software. The screening criteria included: A) risk factors producing >10% change in the regression coefficient after introduction into the basic model; B) parameters with P<0.1 in the regression. All P-values were two-tailed, and values <0.05 were considered statistically significant.
Results
Clinical and laboratory characteristics at biopsy in patients with IgAN-Pso
A total of 85 patients (59 males and 26 females) were selected into this study. The median age was 36 years old (IQR, 29.8- 45.3) and 38.0 years old (IQR, 30.0-46.0) in the serum C3 >0.9 g/l group and the serum C3 <0.9 g/l group, respectively. The median level of eGFR was lower in the low serum C3 group than those in the normal serum C3 group (88.7 ml/min/1.73 m2 [57.6-107] and 76.3 ml/min/1.73 m2 [51.2-102], respectively), without statistical significance. The median levels of proteinuria were comparable. No significant differences were detected in gender, age at biopsy, age at onset of psoriasis, psoriasis duration, blood pressure, serum albumin, triglycerides, uric acid, C-reactive protein, haemoglobin and IgM between the normal serum C3 group and the low serum C3 group. In contrast, significantly lower median levels of cholesterol and serum complement 4 were found in the low serum C3 group, as detailed in Table 1.
| serum C3 >=0.9g/l (n=56) | serum C3 <0.9g/l (n=29) | P-value | |
|---|---|---|---|
| Gender(Male) | 41 (73.21%) | 18 (62.07%) | 0.290 |
| Age at biopsy | 36.0 (29.8-45.3) | 38.0 (30.0-46.0) | 0.522 |
| Age at onset of psoriasis | 26.5 (20.8-33.3) | 27.0 (20.0-31.0) | 0.721 |
| Psoriasis duration | 120.00 (60.0-207) | 120 (84.0-180) | 0.669 |
| Systolic pressure | 128 (117 -138) | 128 (120-137) | 0.937 |
| Diastolic pressure | 80.0 (72.0-85.3) | 80.0 (72.0-89.0) | 0.629 |
| Proteinuria | 1.17 (0.68-1.94) | 1.00 (0.61-1.85) | 0.525 |
| Albumin | 40.6 (37.8-44.4) | 40.5 (37.7-43.2) | 0.985 |
| Cholesterol | 5.02 (4.25-6.11) | 4.55 (3.64-5.17) | 0.048 |
| Triglycerides | 1.63 (1.16-2.38) | 1.43 (1.11-2.00) | 0.423 |
| Serum uric acid | 425 (352-489) | 377.00 (329-407) | 0.058 |
| C-reactive protein | 0.10 (0.10-2.77) | 0.30 (0.10-1.00) | 0.727 |
| Hemoglobin | 132 (118-143) | 127 (113-138) | 0.169 |
| Immunoglobin M | 0.90 (0.55-1.24) | 0.73 (0.59-1.01) | 0.213 |
| Serum complement 4 | 0.24 (0.21-0.29) | 0.18 (0.13-0.20) | <0.001 |
| eGFR | 88.7 (57.6-107) | 76.3 (51.2-102) | 0.211 |
Note: C3: complement 3. eGFR: estimated glomerular filtration rate.
Table 1: Clinical characteristics of the patients with IgA nephropathy secondary to psoriasis at biopsy.
Histological characteristics in patients with IgAN-Pso
The distribution of glomerulus, tubule, interstitial and arterial injuries is shown in Table 2. No statistically differences were noted in the histological characteristics between the two groups. However, more glomeruli developed glomerular sclerosis, segmental sclerosis and crescent in patients with serum C3 <0.9 g/L. Compared to patients with normal serum C3, higher incidence of medium and severe T lesions, E1 lesions, necrosis were observed in patients with low serum C3. Patients in the low serum C3 group had a lower incidence of Interlobular artery lesion without statistical significance.
| serum C3>=0.9g/l (n=56) | serum C3<0.9g/l (n=29) | P-value | |
|---|---|---|---|
| Glomerular sclerosis | 14.3 (6.70-29.6) | 21.00 (10.0-40.0) | 0.237 |
| Mesangial hypercellularity | 21 (37.50%) | 9 (31.03%) | 0.554 |
| Endocapillary hypercellularity | 8 (14.29%) | 6 (20.69%) | 0.450 |
| Segmental sclerosis | 5.75 (0.00-10.65) | 9.10 (0.00-13.90) | 0.167 |
| Segmental sclerosis without adhesion | 42 (75.00%) | 21 (72.41%) | 0.796 |
| T lesion | 0.098 | ||
| <25% | 38 (67.86%) | 18 (62.07%) | |
| 25-49% | 17 (30.36%) | 7 (24.14%) | |
| >=50% | 1 (1.79%) | 4 (13.79%) | |
| Crescent | 0.00 (0.00-8.40) | 3.00 (0.00-7.10) | 0.775 |
| Crescent lesion | 0.270 | ||
| 0 | 31 (55.36%) | 14 (48.28%) | |
| Less than 25% | 21 (37.50%) | 15 (51.72%) | |
| 25% or more | 4 (7.14%) | 0 (0.00%) | |
| Necrosis | 7 (12.50%) | 4 (13.79%) | 0.866 |
| Interlobular artery | 19 (33.93%) | 7 (24.14%) | 0.353 |
Note: IgAN-Pso: immunoglobulin A nephropathy secondary to psoriasis; C3: complement 3; T lesions: tubular atrophy/interstitial fibrosis.
Table 2: Pathological characteristics of the patients with IgAN-Pso.
Univariate analysis related to eGFR
We use eGFR as dependent variable and other variables as independent variable to explore which variables are related to eGFR. All variables were adjusted for gender and age at biopsy. Univariate analysis showed that systolic pressure (β =-0.5, 95%CI -0.9 to -0.1), hemoglobin (β =0.42, 95%CI 0.17 to 0.68), serum uric acid (β =-0.14, 95%CI -0.21 to -0.08), glomerular sclerosis (β =-0.89, 95%CI -1.17 to -0.61), crescent ≥ 25% (β =-43.1, 95%CI -72.5 to -13.8) and T lesions (25-49%: β =-26.6, 95%CI -38.8 to -14.5; ≥ 50%,β =-46.9, 95%CI -70.1 to -23.8) were correlated with eGFR. A positive relationship was found between the levels of serum C3 and eGFR (β =-26.4, 95%CI: -3.4 to 56.1), P=0.086 (Table 3).
| β | 95% CI | P | |
|---|---|---|---|
| Psoriasis duration (months) | -0.05 | (-0.12, 0.02) | 0.185 |
| Age at onset of psoriasis (years) | 0.79 | (-0.08, 1.65) | 0.080 |
| Systolic pressure (mmHg) | -0.50 | (-0.90, -0.10) | 0.016 |
| Diastolic pressure (mmHg) | -0.59 | (-1.19, 0) | 0.054 |
| Proteinuria (g/24h) | -0.34 | (-2.28, 1.59) | 0.730 |
| Albumin (g/L) | 0.83 | (-0.17, 1.83) | 0.109 |
| Cholesterol (mmol/L) | -0.91 | (-4.83, 3.01) | 0.651 |
| Triglycerides (mmol/L) | -2.04 | (-5.72, 1.64) | 0.281 |
| Hemoglobin (g/L) | 0.42 | (0.17, 0.68) | 0.002 |
| Immunoglobin M (g/L) | 9.5 | (-4.27, 23.3) | 0.180 |
| Serum complement 3 (g/L) | 26.4 | (-3.40, 56.1) | 0.086 |
| Serum complement 4 (g/L) | -41.7 | (-128, 44.7) | 0.347 |
| Serum uric acid (umol/L) | -0.14 | (-0.21, -0.08) | <0.001 |
| C reactive protein (mg/L) | -0.38 | (-1.18, 0.43) | 0.362 |
| Glomerular sclerosis (%) | -0.89 | (-1.17, -0.61) | <0.001 |
| Mesangial hypercellularity (n, %) | |||
| No | Reference | - | |
| Yes | -3.5 | (-16.6, 9.57) | 0.601 |
| Crescent lesion (n, %) | |||
| 0 | Reference | ||
| less than 25% | -10.9 | (-23.1, 1.35) | 0.085 |
| 25% or more | -43.1 | (-72.5, -13.8) | 0.005 |
| Endocapillary hypercellularity (n, %) | |||
| No | Reference | - | |
| Yes | -3.56 | (-20.7, 13.6) | 0.685 |
| Segmental sclerosis without adhesion (n, %) | |||
| No | Reference | - | |
| Yes | -10.4 | (-24.5, 3.77) | 0.154 |
| Tubular atrophy/interstitial fibrosis (n,%) | |||
| <25% | reference | ||
| 25-49% | -26.6 | (-38.8, -14.5) | <0.001 |
| >=50% | -46.9 | (-70.1, -23.8) | <0.001 |
| Interlobular artery (n, %) | |||
| No | Reference | - | |
| Yes | -4.98 | (-19.2, 9.27) | 0.495 |
| Necrosis (n,%) | |||
| No | Reference | - | |
| Yes | -6.73 | (-25.4, 11.9) | 0.481 |
All variables were adjusted for gender and age at biopsy.
Table 3: The result of univariate analysis.
The results of relationship between serum C3 and eGFR
We performed covariates screening using Empower Stats software firstly. Variables either producing >10% change in the regression coefficient after introduction into the basic model or with P <0.1 in the regression were adjusted in multivariate analysis. In addition, four regression models were established to explore the association between serum C3 and eGFR, and to prove the stability of the relationship. Serum C3 did not show a statistically significant correlation with eGFR in the non-adjusted model or the adjusted I model (adjusted for basic parameters, including gender and age at biopsy). In the adjust II model, basic parameters and the clinical parameters (including psoriasis duration, age at onset of psoriasis, systolic pressure, diastolic pressure, proteinuria, albumin, cholesterol, triglycerides, hemoglobin, IgM, serum complement 4, serum uric acid and C-reactive protein) were adjusted, serum C3 showed positive correlation (β =72.3, 95%CI: 33.0 to 112; P=0.001) with eGFR. The correlation still existed in the adjust III model (β =72.6, 95%CI: 35.8 to 109; P<0.001), in which the basic, clinical and pathological parameters (glomerular sclerosis, M lesion, E lesion, S-alone lesion, E lesion, T lesion and interlobular artery lesion) were all adjusted. For the purpose of sensitivity analysis, we also handled serum C3 as Categorical variable (Tertile or normal level and low level), and found the positive connection still existed (Table 4).
| Exposure | Non-adjusted | Adjust I | Adjust II | Adjust III |
|---|---|---|---|---|
| Serum C3(total) | 17.8 (-16.2,51.7) 0.308 | 26.4 (-3.40,56.1) 0.086 | 72.3 (33.0,112) 0.001 | 72.6 (35.8,109) <0.001 |
| Serum C3 tertile | ||||
| Low | Reference | Reference | Reference | Reference |
| Medium | 12.5 (-4.93,29.8) 0.164 | 10.3 (-4.96,25.5)0.190 | 25.5 (10.6,40.4) 0.001 | 14.6 (0.04,29.1) 0.055 |
| High | 13.2 (-4.07, 30.4)0.138 | 15.7 (0.58, 30.7) 0.045 | 41.6 (22.6, 60.7) <0.0001 | 33.1 (14.5, 51.6) 0.001 |
| Serum C3<0.9 g/l | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | -10.5 (-25.4, 4.37) 0.170 | -11.3 (-24.3, 1.71) 0.092 | -27.8 (-42.8, -12.8) 0.001 | -17.2 (-32.0, -2.47) 0.026 |
Note: Data were expressed as β(95% confidence interval) P value; C3: complement 3; Non-adjusted model adjust for: none; Adjust I model adjust for: gender; age at biopsy; Adjust II model adjust for: gender; age at biopsy; psoriasis duration; age at onset of psoriasis; systolic pressure; diastolic pressure; proteinuria; albumin; cholesterol; triglycerides; hemoglobin; immunoglobin M; serum complement 4; serum uric acid; C-reactive protein; Adjust III model adjust for: gender; age at biopsy; psoriasis duration; age at onset of psoriasis; systolic pressure; diastolic pressure; proteinuria; albumin; cholesterol; triglycerides; hemoglobin; immunoglobin M; serum complement 4; serum uric acid; C-active protein; glomerular sclerosis; crescent lesion; segmental sclerosis without adhesion; tubular atrophy/interstitial fibrosis; interlobular artery; mesangial hypercellularity; endocapillary hypercellularity.
Table 4: Relationship between the serum complement 3 and eGFR in different models.
The results of stratified analysis
Stratified analysis was firstly performed in order to accurately study the relationship between serum C3 and eGFR, and to exclude the influence factors that have an effect on their relationship. Continuous variables, such as psoriasis duration, age at onset of psoriasis, proteinuria, cholesterol, triglycerides, hemoglobin, IgM, serum complement 4 and serum uric acid, were divided into 2 groups according to the quartiles. The cutoff value of diastolic pressure and systolic pressure were 90 and 140mmHg, respectively, and they were 35 g/L, 10 mg/L, 5%, 25% and 50% for serum albumin, C-reactive protein, crescent, tubular atrophy/interstitial fibrosis and glomerular sclerosis lesions, respectively. All variables were adjusted for gender and age at biopsy.
A positive relationship was perceived between serum C3 and eGFR in patients without E lesions, but it was a negative correlation in patients with E lesions. In patients with S-alone lesions, a positive relationship was seen between serum C3 and eGFR, but the relationship became negative in the S-alone absence group. Interaction tests were carried out to detect the influence of each stratified factor on the relationship between serum C3 and eGFR. No effect modifiers of serum C3 and eGFR were observed (Table 5).
| Variables | N | β (95% CI) | P | P interaction |
|---|---|---|---|---|
| Psoriasis duration | 0.656 | |||
| 1 - 96 | 39 | 8.32 (-36.2, 52.8) | 0.716 | |
| 120 - 490 | 46 | 43.4 (1.67, 85.1) | 0.048 | |
| Age at onset of psoriasis | 0.251 | |||
| 6 - 26 | 42 | 41.7 (-3.86, 87.3) | 0.081 | |
| 27 - 67 | 43 | 12.3 (-26.7, 51.3) | 0.540 | |
| Diastolic pressure | 0.565 | |||
| <90 | 67 | 25.6 (-10.2, 61.4) | 0.166 | |
| >=90 | 18 | 17.4 (-42.2, 76.9) | 0.576 | |
| Systolic pressure | 0.871 | |||
| <140 | 65 | 21.5 (-13.8, 56.7) | 0.237 | |
| >=140 | 20 | 37.2 (-21.4, 95.8) | 0.231 | |
| Proteinuria | 0.659 | |||
| 0.14 - 1.1 | 42 | 29.6 (-10.6, 69.8) | 0.157 | |
| 1.11 - 24.41 | 43 | 26.2 (-16.4, 68.7) | 0.235 | |
| Serum albumin | 0.580 | |||
| <35g/L | 16 | 53.8 (-50.7, 158) | 0.333 | |
| >=35g/L | 69 | 13.1 (-16.6, 42.8) | 0.390 | |
| Cholesterol | 0.340 | |||
| 2.64 - 4.67mmol/L | 42 | 18.3 (-30.0, 66.6) | 0.462 | |
| 4.75 - 13.57 mmol/L | 42 | 41.9 (6.14, 77.7) | 0.027 | |
| Triglycerides | 0.211 | |||
| 0.53 - 1.58 mmol/L | 42 | 52.2 (3.72, 101) | 0.042 | |
| 1.62 - 10.48 mmol/L | 42 | 11.8 (-26.3, 50.0) | 0.547 | |
| Hemoglobin | 0.456 | |||
| 14.5 - 130 g/L | 42 | 41.2 (-4.98, 87.5) | 0.088 | |
| 131 - 177 g/L | 43 | 3.68 (-32.0, 39.3) | 0.841 | |
| Immunoglobin M | 0.652 | |||
| 0.01 - 0.855 g/L | 41 | 27.9 (-19.9, 75.7) | 0.2602 | |
| 0.857 - 2.37 g/L | 41 | 11.8 (-26.2, 49.7) | 0.548 | |
| Serum complement 4 | 0.162 | |||
| 0.1 - 0.221 g/L | 42 | 81.6 (33.5, 130) | 0.002 | |
| 0.223 - 0.44 g/L | 43 | 4.31 (-40.4, 49.0) | 0.851 | |
| Serum uric acid group | 0.845 | |||
| 211 – 398 umol/L | 42 | 19.5 (-18.6, 57.7) | 0.321 | |
| 400 - 683 umol/L | 42 | 49.2 (6.28, 92.0) | 0.031 | |
| C-reactive protein | 0.472 | |||
| <10mg/L | 74 | 37.4 (4.76, 70.0) | 0.028 | |
| >=10mg/L | 11 | 9.62 (-92.4, 112) | 0.859 | |
| Interlobular artery lesion | 0.892 | |||
| No | 59 | 19.0 (-17.5, 55.5) | 0.312 | |
| Yes | 26 | 34.9 (-21.8, 91.6) | 0.240 | |
| Segmental sclerosis without adhesion | 0.087 | |||
| No | 22 | -33.5 (-89.7, 22.8) | 0.259 | |
| Yes | 63 | 31.6 (-4.16, 67.3) | 0.089 | |
| Mesangial hypercellularity | 0.643 | |||
| No | 55 | 29.6 (-4.46, 63.6) | 0.095 | |
| Yes | 30 | 18.2 (-41.0, 77.4) | 0.553 | |
| Endocapillary hypercellularity | 0.829 | |||
| No | 71 | 32.0 (0.88, 63.0) | 0.048 | |
| Yes | 14 | -51.7 (-169, 65.9) | 0.409 | |
| Crescent | 0.162 | |||
| <5% | 54 | 13.5 (-25.1, 52.1) | 0.495 | |
| >=5% | 31 | 27.8 (-18.9, 74.5) | 0.254 | |
| Tubular atrophy/interstitial fibrosis | 0.665 | |||
| < 25% | 56 | 27.8 (-4.73, 60.4) | 0.010 | |
| >=25% | 29 | 24.3 (-22.0, 70.6) | 0.314 | |
| Glomerular sclerosis | 0.286 | |||
| <50% | 74 | 14.8 (-13.7, 43.3) | 0.311 | |
| >=50% | 11 | 74.0 (-14.0, 162) | 0.143 | |
Note: T lesions: tubular atrophy/interstitial fibrosis; Continuous variables, such as psoriasis duration, age at onset of psoriasis, proteinuria, cholesterol, triglycerides, hemoglobin, immunoglobin M, serum complement 4 and serum uric acid, were divided into two groups according to the quartiles. The cutoff value of diastolic pressure and systolic pressure were 90 and 140mmHg, respectively, and they were 35g/L, 10mg/L, 5%, 25% and 50% for serum albumin, C-reactive protein, crescent, tubular atrophy/interstitial fibrosis and glomerular sclerosis lesions, respectively; All variables were adjusted for gender and age at biopsy.
Table 5: Stratified analysis of all variables.
The analyses of non-linear relationship (curve fitting of serum C3 and eGFR)
Curve fitting was utilized to examine the association between serum C3 and eGFR. First, we only adjusted the basic parameters, and then observed the curve relationship between serum C3 and eGFR (Figure 2A). Second, we adjusted the basic parameters and the clinical parameters, and then observed the curve relationship between serum C3 and eGFR (Figure 2B). Third, the basic parameters and the pathological parameters were adjusted, and the curve was likely a straight line (Figure 2C). Finally, all the elementary parameters, clinical and pathological parameters were adjusted, and then a non-linear positive correlation was observed between serum C3 and eGFR (Figure 2D).
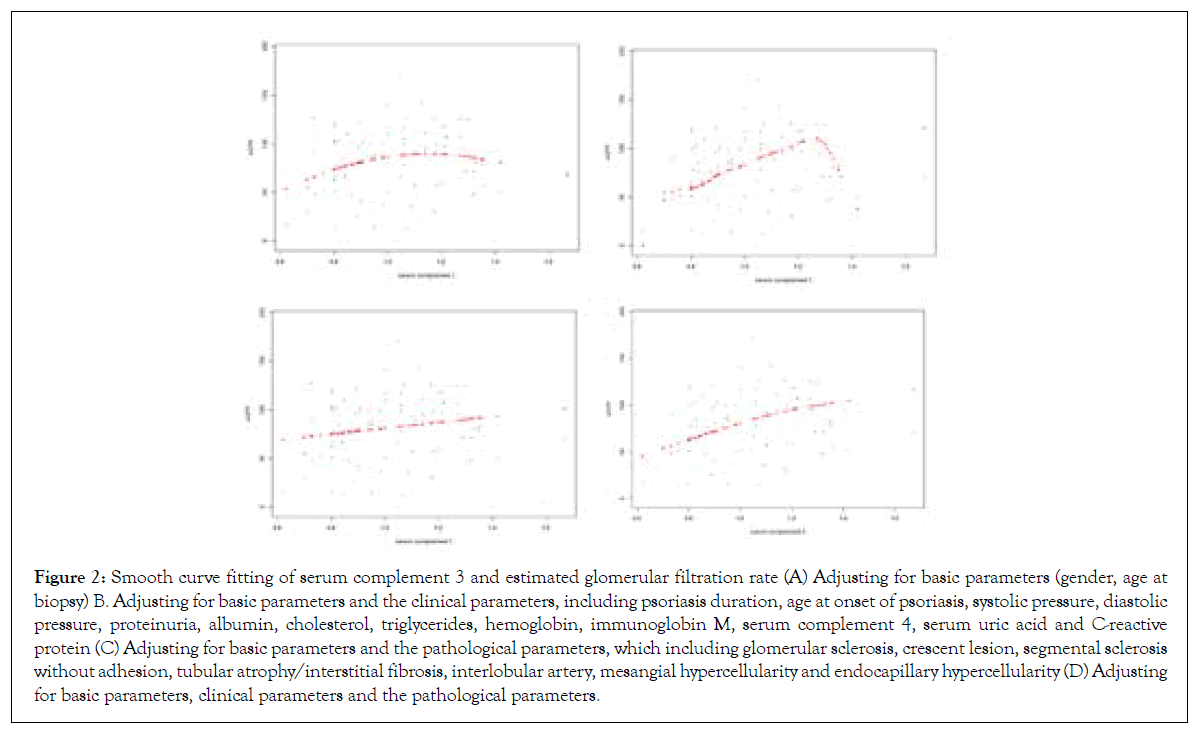
Figure 2: Smooth curve fitting of serum complement 3 and estimated glomerular filtration rate (A) Adjusting for basic parameters (gender, age at biopsy) B. Adjusting for basic parameters and the clinical parameters, including psoriasis duration, age at onset of psoriasis, systolic pressure, diastolic pressure, proteinuria, albumin, cholesterol, triglycerides, hemoglobin, immunoglobin M, serum complement 4, serum uric acid and C-reactive protein (C) Adjusting for basic parameters and the pathological parameters, which including glomerular sclerosis, crescent lesion, segmental sclerosis without adhesion, tubular atrophy/interstitial fibrosis, interlobular artery, mesangial hypercellularity and endocapillary hypercellularity (D) Adjusting for basic parameters, clinical parameters and the pathological parameters.
Discussion
In this investigation, we got a considerable association between serum C3 and GFR in patients with IgAN-Pso. Serum C3 levels are significantly correlated with eGFR in the multivariate models adjusted for potential confounding factors. The eGFR in patients with low C3 decreased by 27.8 ml/min/1.73 m2 and 17.2 ml/ min/1.73 m2 compared with those in patients with normal C3 levels in the adjustment II and adjustment III model, respectively. In addition, we also found that the relationship between serum C3 and eGFR is a nonlinear-positive correlation via further curve fitting.
Before our study, there was no published information concerning the association between serum C3 and GFR in sIgAN, including IgAN-Pso. However, complement activation was involved in both psoriasis and primary immunoglobulin A nephropathy (pIgAN) [11,14,27].
Complement activation is considered to play a pivotal role in the pathogenesis of pIgAN [14]. Complement cloud be activated by the immune complex consisting of galactose-deficient IgA1 (Gd- IgA1) via the alternative, lectin and terminal pathways [19,28]. Zhu B et al. observed low C3 was present in 22.07% in patients with pIgAN, and patients with low C3(less than 79 mg/dL) had severe mesangial hypercellularity than patients with normal serum C3 and C4, however, the renal survival curve did not differ considerably [29]. Komatsu H et al. observed that serum C3 was lower in the severe IgAN patients than mild IgAN patients, but did not differ significantly. Serum IgA/C3 increased in parallel with the histological severity of IgAN [30]. In the present study, the serum C3 was found to be correlated with eGFR after adjustment for confounding factors, but there were no differences in histological characteristics between the serum C3>0.9 g/L group and the C3<0.9 g/L group. Serum C3 was not significantly associated with the histological parameters when we explore the relationship between serum C3 and MEST lesions and the interlobular artery lesions (data not provided).
Although local complement is deemed to be activated in the pathogenesis of psoriatic skin injury, but the role of systemic complement was controversial in psoriasis [11]. On one hand, elevated levels of complement components complement split products and regulator proteins were observed in the serum and plasma of psoriasis patients, indicating involvement of systemic complement activation [31,32]. On the other hand, Fleming et al. found that serum C5b-9 levels were elevated in the absence of immune complexes, such as C1r-C1s-C1-INH and C3bBbP, suggesting that systemic complements are not activated in psoriasis [33]. Chimenti MS et al. reported that C3 and C4 were higher in patients with psoriasis arthritis than the healthy controls, and they were significantly reduced to normalization after anti-TNF therapy, accompanied by a significant reduction of disease activity. However, the cleavage fragments of C3 and factor B were not detected in the patients at baseline, nor at 22 weeks [34]. Low C3 has not been described in patients with psoriasis or psoriasis arthritis, but it was not a rare phenomenon in IgAN-Pso patients as described in the present study. Low serum C3 might be prone to occur in psoriasis patients who developed IgAN as noted by this study. Future studies need to explore the relationship between systemic complement activation and psoriasis and its comorbidities.
Psoriasis is considered an autoimmune disease resulting in an apparent inflammatory disorder. Both the innate and adaptive immune systems play roles in the pathophysiology of psoriasis [35,36]. Complement activation might be a major factor linking innate auto-inflammatory and adaptive autoimmune responses in psoriasis [11]. In a study conducted by Schonthaler et al., C3 was up-regulated by S100A9 (also called calprotectin) when S100A9 was genetically deleted in skin inflammation mouse models, in which psoriasis-like skin disease and inflammation were strongly attenuated, with mild immune infiltrate and decreased amounts of C3. In addition, the inhibition of C3 in the mouse model strongly reduced the occurrence of inflammatory skin disease.
Although psoriasis has been demonstrated to increase the risk of IgAN, little was known about the characteristics of IgAN-Pso, and the underlying mechanism of IgAN-Pso remains elusive. Some researchers have suggested that the association between psoriasis and IgAN might be immune-mediated [37]. Antigliadin IgA was positive in the serum of patients with psoriatic arthritis [38], and it was positive in patients with pIgAN [39]. Complement activation might be one of the causes of IgAN in patients with psoriasis, accounting that complement was involved in the mechanism of psoriasis and pIgAN, as we have discussed detailed before. It was unknown the association between Gd-IgA1 and IgAN-Pso. Cassol et al. reported positive Gd-IgA1 in the renal biopsy samples of sIgAN [40]. Gd-IgA1 was observed in patients with Henoch- Schönlein purpura nephritis [41]. However, the glycosylation of IgA in patients with psoriasis increased as a result of stimulation of the immune system by oxidative stress [42]. This is a different form from Gd-IgA1, which plays an important part in the pathogenesis of pIgAN [43-45]. This may suggest a different mechanism in the pathogenesis of IgAN-Pso. Toll-Like Receptors (TLRs) might participate in the pathogenesis mechanism of IgAN secondary to psoriasis. TLR9 activation could induce overproduction of Gd- IgA1 via APRIL- and IL-6–mediated pathways in ddY mice model [46]. In addition, Ren et al. reported that TLRs/NF-κB (mainly TLR2 and TLR4) pathway was involved in the pathogenesis of renal damage induced by psoriasis-like inflammation. They observed mesangial proliferative lesion, but they did not provide the immunofluorescence staining results [47]. The role of TLRs in IgAN-Pso needs further research. Animal models were urgent to be prepared to investigate the roles of Gd-IgA1, TLRs and complement in the pathogenesis of IgAN-Pso.
One limitation of our study was the retrospective study design. The diagnosis and classification of psoriasis in our study relied on records collected by physicians, in which the classification information was incomplete, and the severity index score of psoriasis were not available. Thus, the impact of the different types and disease activity on the association of serum C3 and GFR were not investigated. A second limitation was the fact that we did not detect the complement split products due to the lack of samples, and were unable to provide more reliable evidence of systemic complement activity in IgAN-Pso. A third limitation was the fact that we did not explore the underlying mechanism of IgAN-Pso, for instance, the role of Gd-IgA1. A fourth limitation was that our study was a single-centre experience from a large tertiary referral centre. Future well-designed, prospective, multicentre clinical research and basic studies are needed to further explore the roles of systemic complement on renal damage in IgAN-Pso.
Conclusion
In conclusion, our study showed that decreased serum C3 was associated with poor eGFR level. In addition, this relationship is non-linear and positive correlation, suggesting that systemic complement activation might be involved in the pathogenesis of IgAN-Pso.
Conflicts of Interest
Da-feng He, Rong Wang, Chun-lei Lu, Shi-jun Li, Chang-hua Liu, Cai-hong Zeng and Zheng Tang declare that they have no conflict of interest.
Informed Consent
Due to the retrospective nature of the study, written informed consent for participation in the study was waived.
Acknowledgements
The authors thank the assistance in kidney pathology diagnosis of Shao-shan Liang, Dan-dan Liang and Feng Xu.
REFERENCES
- Boehncke W-H,Schön MP. Psoriasis. The Lancet. 2015;386 (9997):983-994.
- Mahil SK, Capon F,Barker JN. Genetics of psoriasis. Dermatol Clin. 2015;33(1):1-11.
- Pollock RA, Abji F, Gladman DD. Epigenetics of psoriatic disease: A systematic review and critical appraisal. J Autoimmun. 2017;78:29-38.
- Chiu HY, Huang HL, Li CH, Yin YJ, Chen HA, Hsu ST, et al. Increased risk of glomerulonephritis and chronic kidney disease in relation to the severity of psoriasis, concomitant medication, and comorbidity: a nationwide population-based cohort study. Br J Dermatol. 2015;173(1):146-154.
- Chi CC, Wang J, Chen YF, Wang SH, Chen FL,Tung TH. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: A nationwide population-based cohort study. J Dermatol Sci. 2015;78(3):232-238.
- Wan J, Wang S, Haynes K, Denburg MR, Shin DB,Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961.
- Nakamura-Wakatsuki T, Kato Y, Sakurai K, Yamamoto T. A case of severe erythrodermic psoriasis associated with IgA nephropathy. Int J Dermatol. 2013;52(12):1579-1581.
- Zadrazil J, Tichý T, Horák P, Nikorjaková I, Zíma P, Krejcí K, et al. IgA nephropathy associated with psoriasis vulgaris: a contribution to the entity of 'psoriatic nephropathy'. J Nephrol. 2006;19(3):382-386.
- Grewal SK, Wan J, Denburg MR, Shin DB, Takeshita J, Gelfand JM. The risk of IgA nephropathy and glomerular disease in patients with psoriasis: A population based cohort study. Br J Dermatol. 2017;176(5):1366–1369.
- Saha MK, Julian BA, Novak J, Rizk DV. Secondary IgA nephropathy. Kidney Int. 2018;94(4):674-681.
- Giang J, Seelen MAJ, van Doorn MBA, Rissmann R, Prens EP, Damman J. Complement Activation in Inflammatory Skin Diseases. Front Immunol. 2018;9:639.
- Schonthaler HB, Guinea-Viniegra J, Wculek SK, Ruppen I, Ximenez-Embun P, Guio-Carrion A, et al. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity. 2013;39(6):1171-1181.
- Giacomassi C, Buang N, Ling GS, Crawford G, Cook HT, Scott D, et al. Complement C3 exacerbates imiquimod-induced skin inflammation and psoriasiform dermatitis. J Invest Dermatol. 2017;137(3):760-763.
- Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, et al. Current Understanding of the Role of Complement in IgA Nephropathy. J Am Soc Nephrol. 2015;26(7):1503-1512.
- Kim SJ, Koo HM, Lim BJ, Oh HJ, Yoo DE, Shin DH, et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS ONE. 2012;7(7):e40495.
- Zhang Y, Yan X, Zhao T, Xu Q, Peng Q, Hu R, et al. Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin A nephropathy in mice. Clin Exp Immunol. 2017;189(1):60-70.
- Rops ALWMM, Jansen E, van der Schaaf A, Pieterse E, Rother N, Hofstra J, et al. Interleukin-6 is essential for glomerular immunoglobulin A deposition and the development of renal pathology in Cd37-deficient mice. Kidney Int. 2018;93(6):1356-1366.
- Yamada K, Huang ZQ, Raska M, Reily C, Anderson JC, Suzuki H, et al. Inhibition of STAT3 Signaling Reduces IgA1 Autoantigen Production in IgA Nephropathy. Kidney Int Rep. 2017;2(6):1194-1207.
- Tortjada A, Gutierrez E, Pickering MC,Terente MP, Medjeral-Thomas N. The role of complement in IgA nephropathy. Mol Immunol. 2019;114:123-132.
- Ge YC, Jin B, Zeng CH, Zhang MC, Chen DC, Yin R, et al. PLA2R antibodies and PLA2R glomerular deposits in psoriasis patients with membranous nephropathy. BMC Nephrol. 2016;17(1):185.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496-509.
- Huang X, Wu X, Le W, Hao Y, Wu J, Zeng C, et al. Renal Prognosis and Related Risk Factors for Henoch-Schönlein Purpura Nephritis: A Chinese Adult Patient Cohort. Sci Rep. 2018;8(1).
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612.
- Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546-556.
- Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76(5):534-545.
- Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int. 2017;91(5):1014-1021.
- Ozawa M, Terui T, Tagami H. Localization of IL-8 and Complement Components in Lesional Skin of Psoriasis vulgaris and Pustulosis palmaris et plantaris. Dermatology 2005;211 (3):249-255.
- Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol Res. 2001;167(5):2861-2868.
- Zhu B, Zhu CF, Lin Y, Perkovic V, Li XF, Yang R, et al. Clinical characteristics of IgA nephropathy associated with low complement 4 levels. Ren Fail. 2015;37(3):424-432.
- Komatsu H, Fujimoto S, Hara S, Sato Y, Yamada K, Eto T. Relationship between serum IgA/C3 ratio and progression of IgA nephropathy. Intern Med. 2004;43(11):1023-1028.
- OHKOHCHI K, TORINUKI W, TAGAMI H. Increased plasma concentrations of complement modulating proteins (C1 inhibitor, C4-binding protein, factor H and factor I) in psoriasis. Tohoku J Exp Med. 1988;154(4):315-321.
- Ohkohchi K, Takematsu H, Tagami H. Increased anaphylatoxins (C3a and C4a) in psoriatic sera. Br J Dermatol. 1985;113(2):189-196.
- Fleming CJ, Holme SE, Mackie RM. Systemic complement activation in psoriasis vulgaris. Clin Exp Dermatol. 1996;21(6):415-418.
- Chimenti MS, Ballanti E, Perricone C, Cipriani P, Giacomelli R, Perricone R. Immunomodulation in psoriatic arthritis: focus on cellular and molecular pathways. Autoimmun Rev. 2013;12(5):599-606.
- Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35(6):857-869.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866-873.
- Visconti L, Leonardi G, Buemi M, Santoro D, Cernaro V, Ricciardi CA, et al. Kidney disease and psoriasis: novel evidences beyond old concepts. Clin Rheumatol. 2016;35(2):297-302.
- Lindqvist U, Rudsander A, Bostrom A, Nilsson B, Michaelsson G. IgA antibodies to gliadin and coeliac disease in psoriatic arthritis. Rheumatology. 2002;41(1):31-37.
- Smerud HK, Fellstrom B, Hallgren R, Osagie S, Venge P, Kristjansson G. Gluten sensitivity in patients with IgA nephropathy. Nephrol Dial Transplant. 2009;24(8):2476-2481.
- Cassol CA, Bott C, Nadasdy GM, Alberton V, Malvar A, Nagaraja HN, et al. Immunostaining for galactose-deficient immunoglobulin A is not specific for primary immunoglobulin A nephropathy. Nephrol Dial Transplant. 2020;35(12):2123-2129.
- Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, et al. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schonlein purpura nephritis. Kidney Int. 2011;80(1):79-87.
- Damasiewicz-Bodzek A,Wielkoszynski T. Advanced protein glycation in psoriasis. J Eur Acad Dermatol Venereol. 2012;26(2):172-179.
- Robert T, Berthelot L, Cambier A, Rondeau E, Monteiro RC. Molecular Insights into the Pathogenesis of IgA Nephropathy. Trends Mol Med. 2015;21(12):762-775.
- Suzuki Y, Matsuzaki K, Suzuki H, Okazaki K, Yanagawa H, Ieiri N, et al. Serum levels of galactose-deficient immunoglobulin (Ig) A1 and related immune complex are associated with disease activity of IgA nephropathy. Clin Exp Nephrol. 2014;18(5):770-777.
- Placzek WJ, Yanagawa H, Makita Y, Renfrow MB, Julian BA, Rizk DV, et al. Serum galactose-deficient-IgA1 and IgG autoantibodies correlate in patients with IgA nephropathy. PLoS One. 2018;13(1):e0190967.
- Makita Y, Suzuki H, Kano T, Takahata A, Julian BA, Novak J, et al. TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy. Kidney Int. 2020;97(2):340-349.
- Ren F, Zhang M, Zhang C, Sang H. Psoriasis-Like Inflammation Induced Renal Dysfunction through the TLR/NF-κB Signal Pathway. Biomed Res Int. 2020;3535264.
Citation: He D, Wang R, Lu C, Li S, Liu C, Zeng C, et al. (2021) Association of Low Serum Complement 3 with Worse Glomerular Filtration Rate in Patients with IgA Nephropathy Secondary to Psoriasis. J Vaccines Vaccin. S11: 001.
Copyright: © 2021 He D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : No funding