Indexed In
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 10, Issue 1
Assessment of Dumpsite Soils in Mangrove Forest at Eagle Island, Nigeria: ItâÂÂs Effect on Potential Bioavailability of Heavy Metals in the Environment
Ayobami Aigber1 and Aroloye O Numbere2*2Department of Animal and Environmental Biology, University of Port Harcourt, Choba, Nigeria
Received: 08-Mar-2019 Published: 29-Mar-2019, DOI: 10.35248/2157-7463.19.10.390
Abstract
Heavy metals can be absorbed by plants resulting to contamination of other organisms in the food chain. This study was intended to determine heavy metals in soil, their mobility factor and impact on flora and fauna. To determine bioavailability of metal ions in soil chemical speciation and mobility factor indices were calculated. The level of Fe, Pb, Zn and Cd in readily available forms were assessed in dumpsite soils within mangrove forest in the Eagle Island and compared with control (i.e., relatively undisturbed soil). The aim of the study was to evaluate the impact and contribution of municipal waste discharge on alteration of soil quality and bioavailability of metals within the soil matrix. Samples of soil were collected in triplicate from five locations across the dump area while the control point was established at a less impacted area. Sampling was done in November 2018. Concentration of metal ion/species was analysed using GBC Avanta PM A6600 atomic absorption spectrophotometer. Soils of the sampling area were acidic with pH values ranging between 4.55 and 5.74. The most important heavy metal fractions in the dumpsite soils were; Fe (residual fraction, 53.75%), Pb (residual fraction, 42.58%), Zn (Fe-Mn oxide fraction, 46.85%) and Cd (carbonate bound fraction, 37.77%). However, the less impacted soil was predominantly affiliated to the residual fractions of Fe (68.75%), Pb (54.86%), Zn (37.45%) and Cd (51.51%). Heavy metal mobility factor indices reflected the order: (Cd>Pb>Zn>Fe) for soils of both the solid waste dumpsite and control areas. Despite the prevalence of heavy metals to the inert fractions, the significant affiliation of Cd to the readily mobile fractions of waste dump soils may suggest its release to have come from toxic constituents such as petroleum products that are associated with municipal wastes.
Keywords
Speciation; Mobility; Bioavailability; Eagle Island; Port Harcourt; Mangroves
Introduction
The over-reliance of most communities on the open dump and landfill systems [1] as the primary practise for solid waste management is fast becoming an environmental problem. The soil systems serve as sink for contaminants while heavy metals can persist in soil [2]. Sometimes, the presence of organic matter in soil emanating from municipal wastes can serve as metal chelators whereby they become non-bioavailable in the soil ecosystem. Waste in most areas can be liquid or solid. Liquid waste comes from industrial effluents discharged into the sea by petrochemical or manufacturing industries sited close to coastal areas. In the same vein solid waste are dumped on forest soil leading to contamination and pollution of the soil resulting in the formation of waste land.
The growing number of petrochemical and servicing industries operating within the Port Harcourt metropolis, especially along coastal areas similar to the Eagle Island leads to the proliferation of heavy metal point sources around surrounding water bodies [3,4]. Water run-offs at high tide often lead to the redistribution of dumpsite leachates and solid waste materials along adjoining river estuaries [5].
Solid waste management continues to pose significant problems to most environmental agencies in developing countries [6]. This problem may persist due to unavailable land, continuous environmental deterioration and health effect [7]. Metal ions can form complexes with naturally occurring ligands emanating from industrial processes. They become mobile and transportable in the environment and within biological systems such as mangroves [8]. The effect of various metal complexes been formed is dependent on the species which are kinetically and thermodynamically stable in homogenous and heterogeneous systems [9].
The Eagle Island solid waste dumpsite is located in the Port Harcourt city local government area of Rivers state, Nigeria. Adjoining river estuaries which cut across the area connects to major oil and gas industries in the heart of the Niger Delta region and as such plays host to oil installations and heavy vessel movement for crude oil transportation [10,11]. The river is tidal and sometimes overflows; driving spilt crude and dumpsite leachates into surrounding water bodies [12,13]. Also, other forms of indiscriminate municipal discharges negatively impact on the overall quality of the aquatic environment. The environmental importance of chemical speciation on soil systems around dumpsites of the Eagle Island area is enormous due to the economic importance of the adjoining river which serves as link to many oil and gas industries in the area. The socio-economic impact of improper waste management practices on the soil ecosystem and the attendant adverse environmental impact on the biological systems (food crops and tubers) have led to far reaching consequences such as the abandonment of arable agricultural lands, the consequential pollution of surrounding water bodies and decimation of aquatic biota, poor economic activities, infiltration of contaminants into surrounding groundwater and other associated adverse health effects [14,15].
The five-stage multi-step extraction procedure previously applied by Aigberua and Tarawou [16] was adopted for the present study. The extraction scheme provided a better understanding of the concept of bioavailability and mobility of metal ions in the soil ecosystem. The potential hazard and fate of metals in the soil habitat can be derived from the identification and quantification of different species of the metal ions. The application of mathematical models (such as, mobility factor indices) across soil-available mobile and immobile metal species or forms helps to reveal the potential risk posed by different activities within a soil or sediment environment.
The essence of this work is to determine the fate and effect of open dump systems on increasing toxicity potential and bioavailability of heavy metal micro-pollutants in soils. The objective of this study therefore include: (1) to determine the fractional concentration of heavy metals in soil; (2) to determine the mobility factor indices of metals in waste dump and control soils; (3) to evaluate the relationship between mobility factor of metals and contamination of environmental flora and fauna in study vicinity.
Materials and Methods
Description of study area
Eagle Island is a coastal community surrounded by thick mangrove forest vegetation (Figure 1a and 1b). Anthropogenic activities emanating from increased urban development such as deforestation and building construction has led to the decline in biodiversity and mangrove population. The solid waste dumpsite soil around Eagle Island area is located along the river course, with proximity to a patch of mangrove forests. The river stretch transverses several oil services and petroleum product storage facilities. The field location is characterized by loose sandy soil bound by light vegetation covers. Crude oil seepage into surrounding soil is commonplace within vicinity of the dumpsite as residual oil can be found within the soil environment especially at locations that are close to oil well heads. Mineral oil in soil and other associated toxicants emanating from the dumpsite has become a menace to the people who are mainly fish farmers.
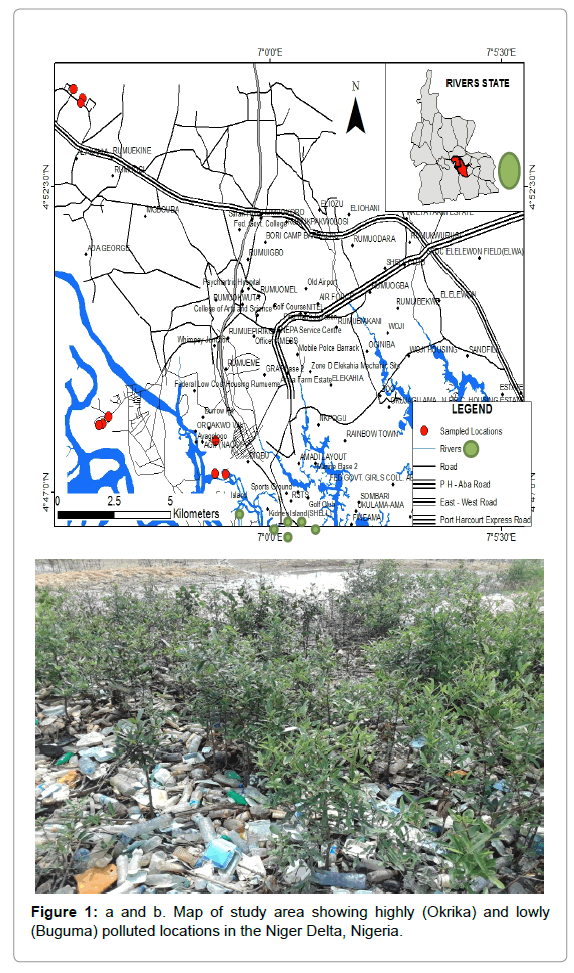
Figure 1: a and b. Map of study area showing highly (Okrika) and lowly (Buguma) polluted locations in the Niger Delta, Nigeria.
Experimental design and field sampling
Soil samples were randomly collected at depths of 0-15 cm from five (5) locations across the solid waste dump area, while one (1) control point with insignificant trace of waste contamination was established at a distance of about 10 m from waste dump (Figure 2). Soil auger was used to collect top soils around the waste dumpsite area and each sampling point was geo-referenced with a hand-held Garmin Etrex model GPS. The recorded coordinates are as follows: site 1 (N 4º47.223´, E6º 58.134´), site 2 (N 4º47.329´, E6º 58.573´), site 3 (N 4º47.327´, E6º 58.573´), site 4 (N 4º47.326´, E6º 58.574´), site 5 (N 4º47.327´, E6º 58.574´), site 6 (control) (N 4º47.325´, E6º 58.565´) (Figure 1a and 1b). Triplicate samples were collected and transferred into pre-cleaned polyethylene bags before been stored in an ice chest.
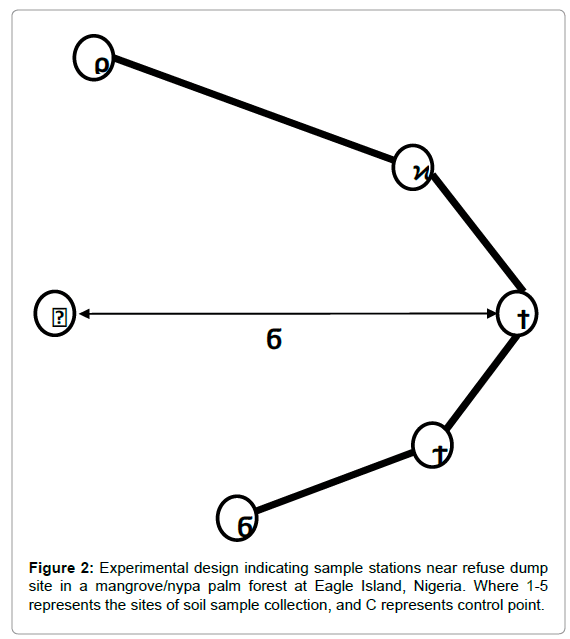
Figure 2: Experimental design indicating sample stations near refuse dump site in a mangrove/nypa palm forest at Eagle Island, Nigeria. Where 1-5 represents the sites of soil sample collection, and C represents control point.
Statistical analysis
In order to determine the association and variation across soil metal forms or fractions and the different heavy metals been studied, statistical analysis was carried by hierarchical cluster analysis (HCA) using Euclidean distance based on average linkage between groups. Also, cluster analysis was carried out for two variables (heavy metals and their different fractions) across soils of the waste dumpsite and the control location. Clustering techniques are used to isolate objects associated with a specific cluster; such objects should be quite similar.
Heavy metals of mutual dependence show similarity or closeness in characteristics while those of mutual independence reflect differing characteristics. Similarly, analysis of variance (ANOVA) was done to determine the significance, and bar graphs were plotted in R Development Core Team.
Sample preparation, analytical validation and quality control procedure
Soil samples were air-dried at room temperature, homogenized after grinding, sieved through a 2 mm mesh size sieve and transferred into plastic vials. Glassware was acid-washed and rinsed under tap water, further rinsed 3 to 4 times with distilled water. Thereafter, they were left to drain water before been stored in a desiccator to dry. Reagent blank was prepared for each metal fraction to be analysed. Working standard solutions of 100 mg/l was prepared from an Accu Standard-USA stock standard solution (1,000 mg/l) of each test metal. Immediately after instrument calibration, method blank was been run for quality control (QC) of the analytical process. Quality control samples were aspirated at the beginning and after each metal run. The following wavelengths were used for the analysis of metals: Fe (372.0 nm), Pb (217.0 nm), Zn (213.9 nm) and Cd (228.8 nm). Analyte recovery was conducted on spiked soil sample. The spiked samples were subjected to similar sample treatment. The results depicted efficiency in the digestion and recovery process. The percentage recovery and coefficient of variation of spiked replicate samples were: (Fe, %R=93.76-97.50%, C.V=0.44- 2.84%); (Pb, %R=88.07-91.89%, C.V=4.02-9.21%); (Zn, %R=89.05- 94.23%, C.V=3.54-7.74%); (Cd, %R=92.03-94.06%, C.V=2.32-6.98%).
Reagents and chemicals
The reagents and chemicals used are of analytical grade, some of which include: 37% hydrochloric acid (Sigma-Aldrich, Germany), 65% nitric acid, 30% hydrogen peroxide and 39%-43% hydrofluoric acid (BDH-Poole, UK). All standard solutions were prepared using distilled water; working standards of the seven metals under study were prepared by diluting different known volumes of their stock solutions of 1,000 mgdm-3.
Sequential extraction of heavy metals
The five-stage sequential extraction scheme reported by Aigberua and Tarawou [16] was used. 1.0 g of air-dried soil was sequentially extracted as follows:
Fraction 1 (Exchangeable metals): 1 ± 0.0001 g duplicate portion of finely divided sample was weighed into centrifuge tubes, 8 ml of 1 M MgCl2.6H2O (pH=7) was added and the soil solution was extracted at room temperature for 1 hour with continuous agitation before been centrifuged at 2500 rpm for 20 minutes. After centrifugation, the mixture was filtered and the supernatant was stored.
Fraction 2 (Metals bound to carbonates): 8 ml of 1 M sodium acetate (pH=5, using acetic acid) was added to the recovered residue from fraction 1 at room temperature and under continuous agitation for 5 hours. Mixture was further centrifuged at 2500 rpm for 20 minutes before storing the supernatant.
Fraction 3 (Metals bound to Fe-Mn oxide): To the residue obtained from fraction 2 was added 20 ml of 0.04 M hydroxylamine hydrochloride (NH2OH.HCl) in 25% v/v acetic acid. The solution was heated in a water bath at 96°C for 6 hours. Afterwards, mixture was centrifuged at 2500 rpm for 20 minutes and supernatant stored.
Fraction 4 (Metals bound to organic matter): 3 ml of 0.02 M nitric acid (HNO3) and 5 ml of 30% hydrogen peroxide (H2O2) (pH=2, using nitric acid) was added to the residue obtained from fraction 3. Mixture was heated in a water bath at 85°C for 3 hours and cooled, a further 5 ml of 1.2 M ammonium acetate in 20% v/v nitric acid (HNO3) was added and diluted to 20 ml with distilled water, mixture was intermittently agitated for 30 minutes prior to a 20 minute centrifugation at 2500 rpm before storing the supernatant.
Fraction 5 (Residual metals): Residue from fraction 4 was transferred into teflon beakers with minimum amount of distilled water before adding 2 ml HCl, 6 ml HNO3 and 3 ml of 30% H2O2. Mixture was digested on a hotplate (in a fume cupboard) until almost dry. Further volumes of HNO3, HF and 30% H2O2 were added and digested on a hot plate to near-dryness. 20 ml of distilled water was added and digested on a hotplate to about 15 ml volume. The extract was diluted to 25 ml with distilled water.
One gram of each soil sample was weighed and sequentially extracted using different selective extraction solutions as applicable for the fractional isolate of each metal. Distilled water was used to wash the residues after each extraction to ensure selective dissolution by the different extraction fluids. All samples were run in triplicate; the analytical scheme applied is stated below.
Results and Discussion
Soil pH level
Soils were mostly acidic rather than alkaline, which may have come from the different pathogenic wastes dumped at the sampling area.
Values of pH units ranged between 4.55 and 5.74 with a mean value of 5.16 ± 0.42 (Table 1). This acidic medium favors heavy metal retention in soil. This result is supported by the report of Numbere [17] where similar values were recorded for soils of a mangrove forest in the Niger Delta region of Nigeria.
| Sample code | pH |
|---|---|
| E1 | 4.89 |
| E2 | 4.55 |
| E3 | 5.06 |
| E4 | 5.28 |
| E5 | 5.45 |
| E6 | 5.74 |
Table 1: Levels of pH in soils collected from Eagle Island.
Fractional concentration of heavy metals in soil
The waste dump soil reflected the range and mean concentrations of exchangeable, carbonate, organic matter and residual bound metal fractions (Table 2). Apart from Zn which depicted higher concentration in the less impacted soil, all the other metals (Fe, Pb and Cd) revealed higher amounts of exchangeable metal species in soils of the waste dump. Apart from Pb and Zn which depicted higher concentrations in the less impacted soil, all the other metals (Fe and Cd) contained more Fe-Mn oxide bound metal species in the waste-impacted soil. All metals were relatively higher in the waste dumpsite when compared to the control location. Similarly, only Fe depicted higher levels in the less impacted soil, all other metals (Pb and Zn) were of higher concentration in soils of the waste dump. In general, the organic matter bound species of Cd was observed below detection limit in both the impacted and control locations. Residual metal ion/species were predominantly higher in the control soil as compared to soils of the waste dumpsite (Table 3).
| Metal fractions | Fe (Mg/Kg ± SE) | Pb (Mg/Kg ± SE) | Zn (Mg/Kg ± SE) | Cd (Mg/Kg ± SE) | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | Range | Mean | Range | Mean | |
| Exchangeable | 60.03-79.82 | 72.58 ± 7.85 | 15.5-37.3 | 24.6 ± 8.5 | 10.2-13.5 | 11.9 ± 1.5 | 0.5-1.2 | 0.71 ± 0.30 |
| Carbonate | 2.58-17.90 | 8.32 ± 5.85 | 16.2-35.2 | 23.9 ± 6.9 | 1.3-3.4 | 2.5 ± 0.8 | 1.2-1.8 | 1.58 ± 0.27 |
| Fe-Mn oxide | 2,962.84-5,022.72 | 3,797.79 ± 933.88 | 32.8-60.1 | 45.1 ± 12.7 | 32.7-61.3 | 50.2 ± 13.6 | 0.001-1.6 | 0.68 ± 0.74 |
| Organic matter | 13.70-3,049.08 | 1,278.9 ± 1,368.2 | 0.001-29.4 | 7.0 ± 12.8 | 0.96-21.9 | 11.9 ± 8.7 | <0.001 | <0.001 |
| Residual | 2,880.53-10,431.40 | 5,995.1 ± 3,044.8 | 30.0-123.8 | 74.5 ± 43.4 | 15.1-51.9 | 30.6 ± 15.5 | 0.001-2.6 | 1.22 ± 1.06 |
Table 2: Range and mean concentration of metal bound fractions in soil from waste dump site at Eagle Island, Niger Delta. Nigeria (where n=5).
| Metal fractions | Fe (Mg/Kg) | Pb (Mg/Kg) | Zn (Mg/Kg) | Cd (Mg/Kg) |
|---|---|---|---|---|
| Exchangeable | 68.75 | 23.66 | 16.12 | 0.02 |
| Carbonate | 7.83 | 32.41 | 2.74 | 1.20 |
| Fe-Mn oxide | 1,210.36 | 20.32 | 30.62 | <0.001 |
| Organic matter | 2,078.96 | <0.001 | 8.48 | <0.001 |
| Residual | 7,405.00 | 92.83 | 34.70 | 1.30 |
Table 3: Range and mean concentration of metal bound fractions in soils from control site at Eagle Island, Niger Delta. Nigeria (where n=1).
Prevailing metal associations within waste dump systems of Eagle Island
The resulting data reveals the heavy metal species or forms which are prevalent in the soil environment. The heavy metal species assessed include: Fe, Pb, Zn and Cd. The metal fractions being evaluated are the mobile and immobile species which can either become bio-available or immobile within the soil ecosystem. Metal fractions were identified in soils of the waste dumpsite and less impacted (control station). Sampling was executed in the month of November 2018 (dry season). A total of six (6) soil samples were collected and analysed using multi-step extraction procedures as adopted by Aigberua and Tarawou [16]. The results are presented in Figures 1-4 for soils of the waste dumpsite and less impacted area.
Generally, the results obtained from this study revealed that all metals were prevalent in the poorly mobile and immobile fractions (Figure 3a-3g). In soils of the waste dump, the most prevalent metal fractions were: Fe (residual fraction, 53.75%), Pb (residual fraction, 42.58%), Zn (Fe-Mn oxide fraction, 46.85%) and Cd (carbonate bound fraction, 37.77%). However, the less impacted soil (control station) was predominantly affiliated to the residual fractions of Fe (68.75%), Pb (54.86%), Zn (37.45%) and Cd (51.51%).
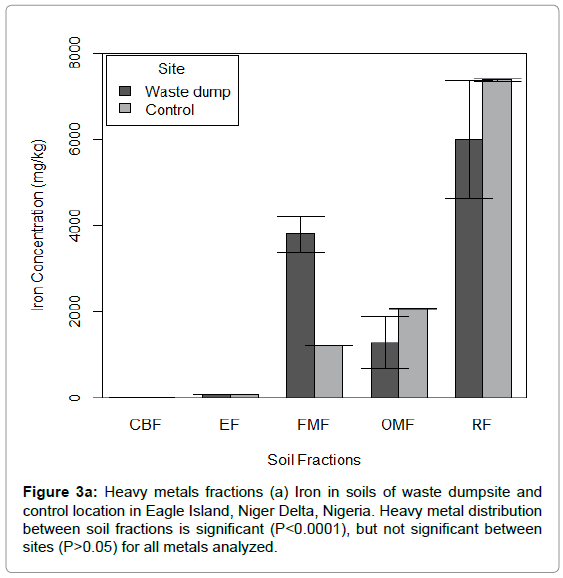
Figure 3a: Heavy metals fractions (a) Iron in soils of waste dumpsite and control location in Eagle Island, Niger Delta, Nigeria. Heavy metal distribution between soil fractions is significant (P<0.0001), but not significant between sites (P>0.05) for all metals analyzed.
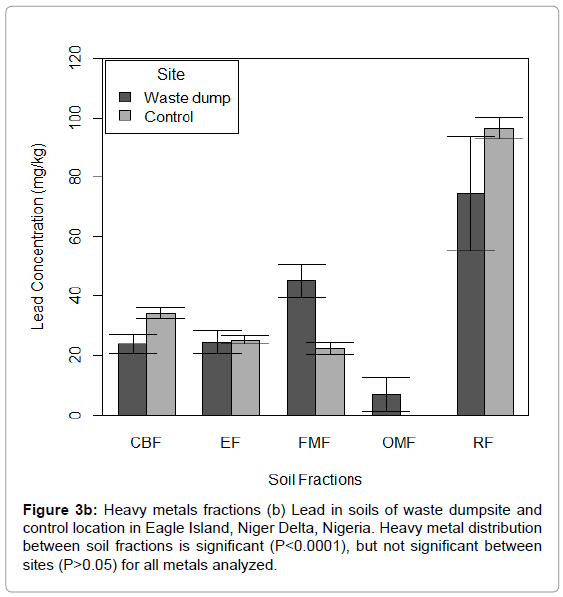
Figure 3b: Heavy metals fractions (b) Lead in soils of waste dumpsite and control location in Eagle Island, Niger Delta, Nigeria. Heavy metal distribution between soil fractions is significant (P<0.0001), but not significant between sites (P>0.05) for all metals analyzed.
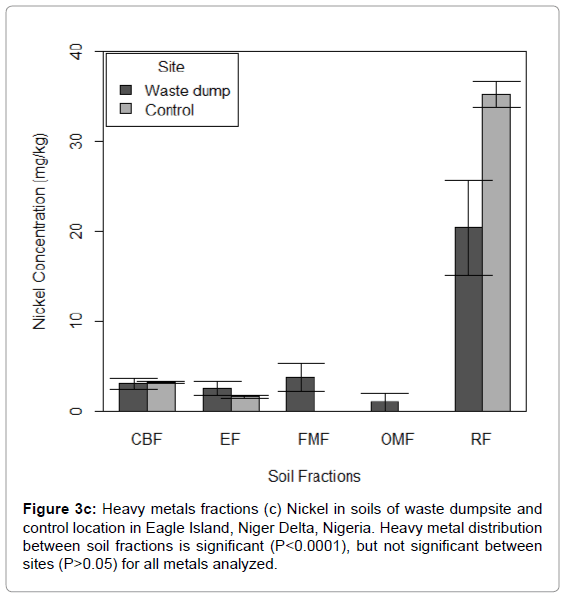
Figure 3c: Heavy metals fractions (c) Nickel in soils of waste dumpsite and control location in Eagle Island, Niger Delta, Nigeria. Heavy metal distribution between soil fractions is significant (P<0.0001), but not significant between sites (P>0.05) for all metals analyzed.
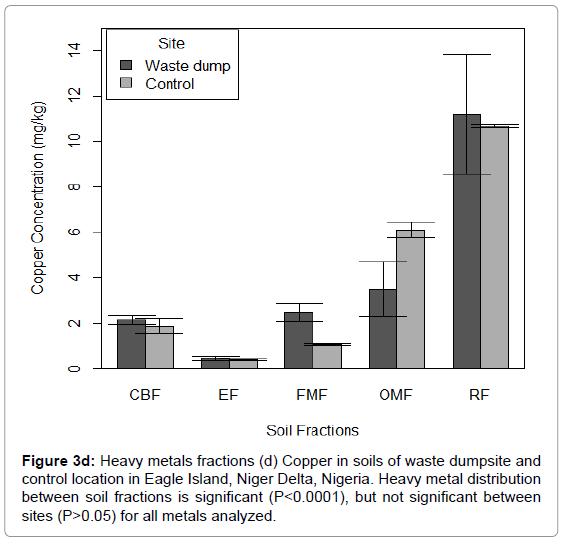
Figure 3d: Heavy metals fractions (d) Copper in soils of waste dumpsite and control location in Eagle Island, Niger Delta, Nigeria. Heavy metal distribution between soil fractions is significant (P<0.0001), but not significant between sites (P>0.05) for all metals analyzed.
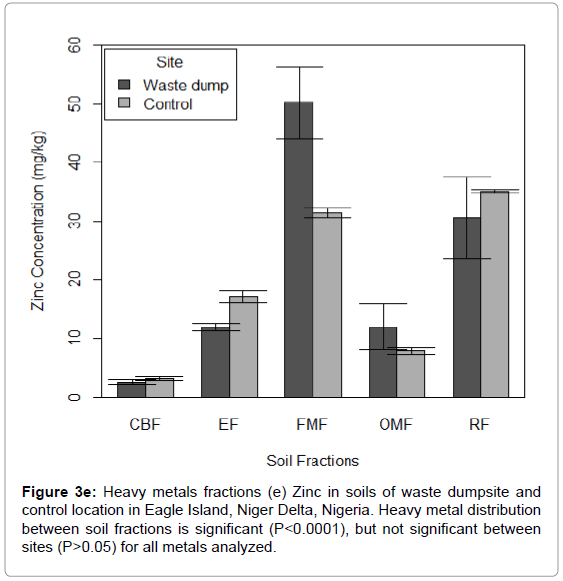
Figure 3e: Heavy metals fractions (e) Zinc in soils of waste dumpsite and control location in Eagle Island, Niger Delta, Nigeria. Heavy metal distribution between soil fractions is significant (P<0.0001), but not significant between sites (P>0.05) for all metals analyzed.
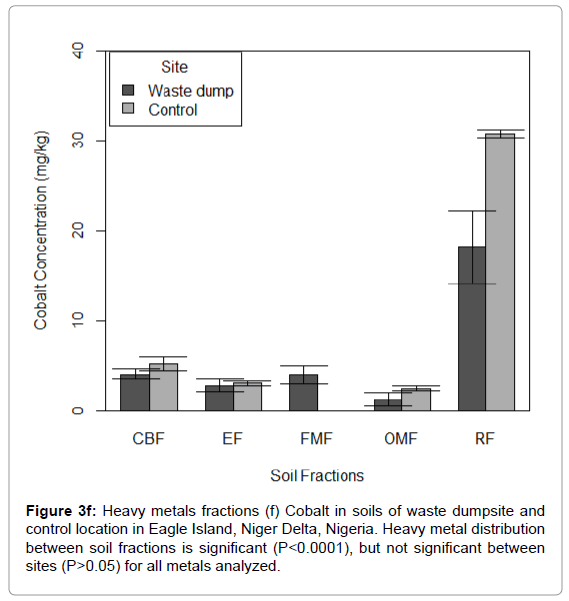
Figure 3f: Heavy metals fractions (f) Cobalt in soils of waste dumpsite and control location in Eagle Island, Niger Delta, Nigeria. Heavy metal distribution between soil fractions is significant (P<0.0001), but not significant between sites (P>0.05) for all metals analyzed.
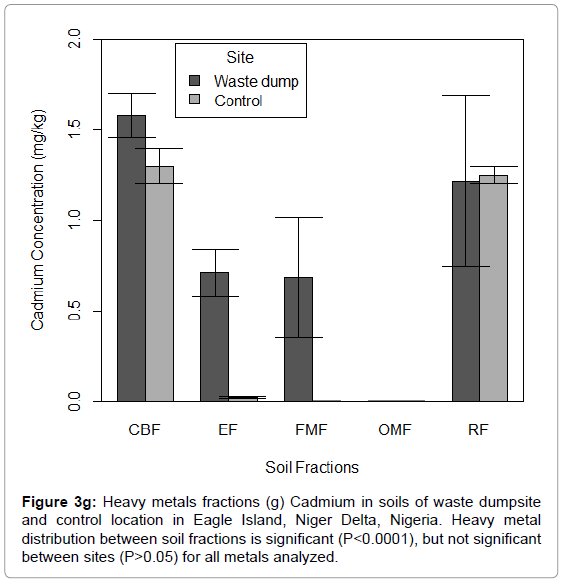
Figure 3g: Heavy metals fractions (g) Cadmium in soils of waste dumpsite and control location in Eagle Island, Niger Delta, Nigeria. Heavy metal distribution between soil fractions is significant (P<0.0001), but not significant between sites (P>0.05) for all metals analyzed.
On the other hand, metal species in control soil was mainly of the non-bioavailable forms. Consequently, materials emanating from the waste dumpsite may have aided the conversion of metals to their bioavailable forms. The ease of transportability of Cd (carbonate-bound species) in soils of the waste dump seems to depict it with most toxicity potential within the study area.
Furthermore, the mobility factor indices for heavy metals revealed the sequence: (Cd>Pb>Zn>Fe) for soils of the waste dump and control locations respectively. Soils collected from the waste dump recorded the following mobility factors: Fe (0.84%), Pb (29.36%), Zn (14.78%) and Cd (61.90%), while the control station depicted the following mobility factors: Fe (0.71%), Pb (33.14%), Zn (20.35%) and Cd (48.57%).
Expectedly, the iron forms in the soil are indigenous to the natural soil system of the mangrove swamp [17,18] as reflected by its poor mobility and preferred association with residual fractions of the soil matrix. Therefore, the presence of leachable toxicants in soils of the waste dump may be responsible for the elevation in potential toxicity of heavy metals in soil (especially Cd) and neighbouring vegetation such as mangrove [17] and nypa palm trees.
Results from this study were similar to levels reported by Nobrega et al. [18] that majority of Fe (29.20%) was affiliated to residual fractions of soils around automobile waste dumpsites.
Mobility factor indices of metals in soils of Eagle Island
The fate of metal ions in soil is dependent on its form which is depicted by the resulting mobility factor indices. Mobility factors (MF) of metals provide an indication of a metals bioavailability or biological inactivity [16,19,20]. Soil metals are fractionated in the order of decreasing solubility; therefore, the exchangeable and carbonate-bound fractions (F1+F2) which are the free and easily available metal ions reflect the level of bio available forms (Figure 4c). The relative index of metal mobility was calculated as a mobility factor (MF) [21] by applying Eqn. 1:
MF=[(F1+F2)÷(F1+F2+F3+F4+F5)] × 100 (1)
F1 (exchangeable fraction), F2 (carbonate-bound fraction), F3 (Fe- Mn oxide bound fraction), F4 (organic matter bound fraction) and F5 (residual fraction).
Definition of variables
The mobility factor of soils from waste dump and control locations represent samples collected in the dry season month of November 2018. The observed sequence for metal mobility factor indices revealed (Cd>Pb>Zn>Fe) for both soils of the waste dump and control locations respectively. Consequently, the significant level of cadmium and lead mobility may be attributed to the impact of poor solid waste management practices within Eagle Island area. Iron and zinc forms in the soil were revealed to be mainly natural constituents of the soil matrix. This is because they are strongly bonded within the interstitial matrices of the soil (as residual fractions). This study was in agreement with Osakwe [19] that reported mobility factor indices of 16.49 to 32.13% for iron, with a mobility factor sequence revealing (Ni>Fe) for soils around automobile waste dumpsites. Similarly, it partially compares with the report of Osakwe et al. [22] where greater mobility factor indices for Cd had been reported when compared to Pb and Fe. This is depicted by the trends (Zn>Cd>Pb>Fe) and (Cd>Zn>Pb>Fe) for bottom sediments of the Imo River system during the dry and wet seasons respectively. Also, this study was in partial agreement with Aigberua and Tarawou [16] where mobility factors for heavy metals reportedly depicted the following trend: Pb>Zn>Cd>Fe and Pb>Cd>Zn>Fe for the sample and control points respectively.
Interactions between heavy metals and their fractions in Eagle Island soil
Figure 4 shows the hierarchical cluster analysis of the association of different metal ion/forms (OMF, CEF, EF, FMF and RF) for waste dumpsite and control and different heavy metals (Pb, Zn, Cd, and Fe) in waste dumpsite soils and control of Eagle Island. EF-exchangeable fraction, CBF-carbonate bound fraction, FMF-Fe-Mn oxide fraction, OMF-organic matter bound fraction, RF-residual fraction, Pb-Lead, Zn-zinc, Cd-cadmium, Fe-iron.
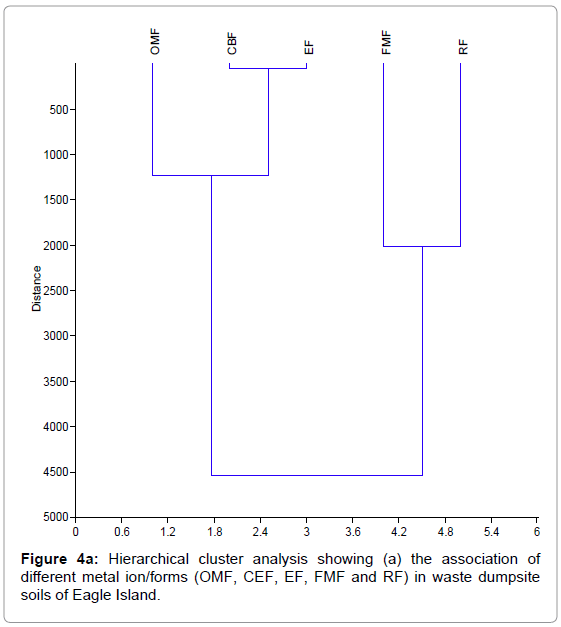
Figure 4a: Hierarchical cluster analysis showing (a) the association of different metal ion/forms (OMF, CEF, EF, FMF and RF) in waste dumpsite soils of Eagle Island.
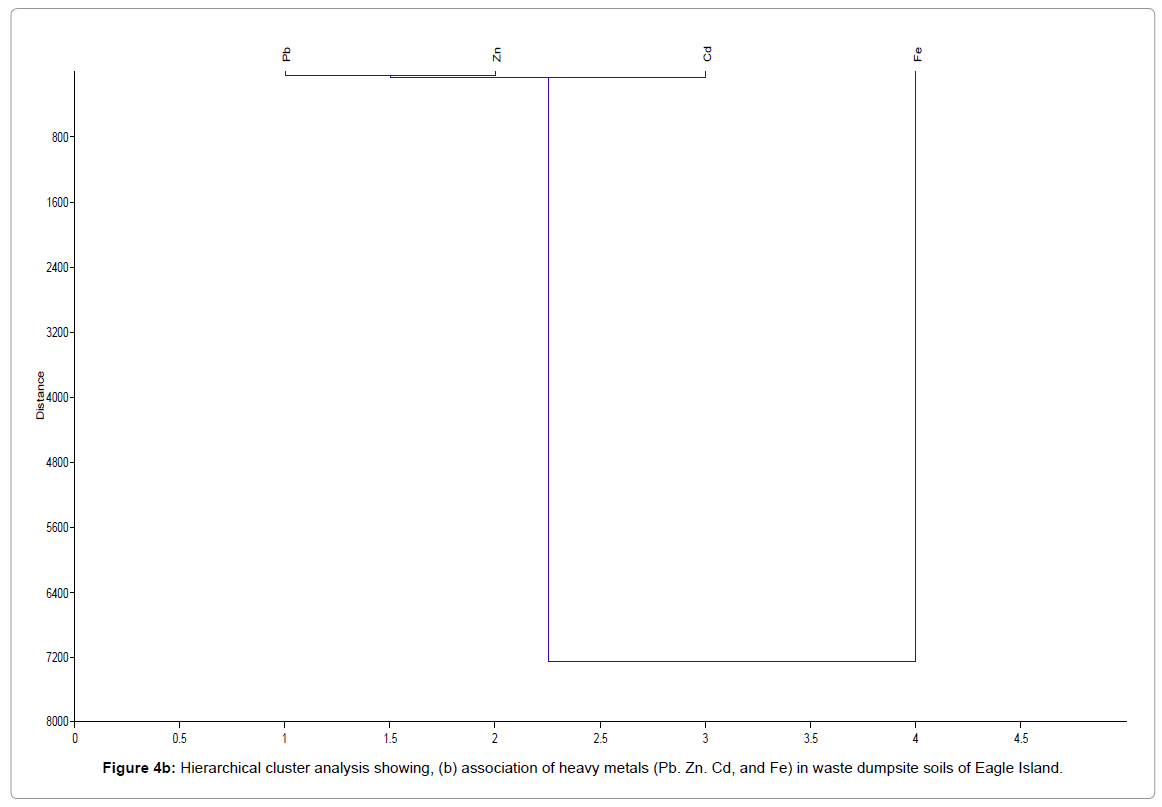
Figure 4b: Hierarchical cluster analysis showing, (b) association of heavy metals (Pb. Zn. Cd, and Fe) in waste dumpsite soils of Eagle Island.
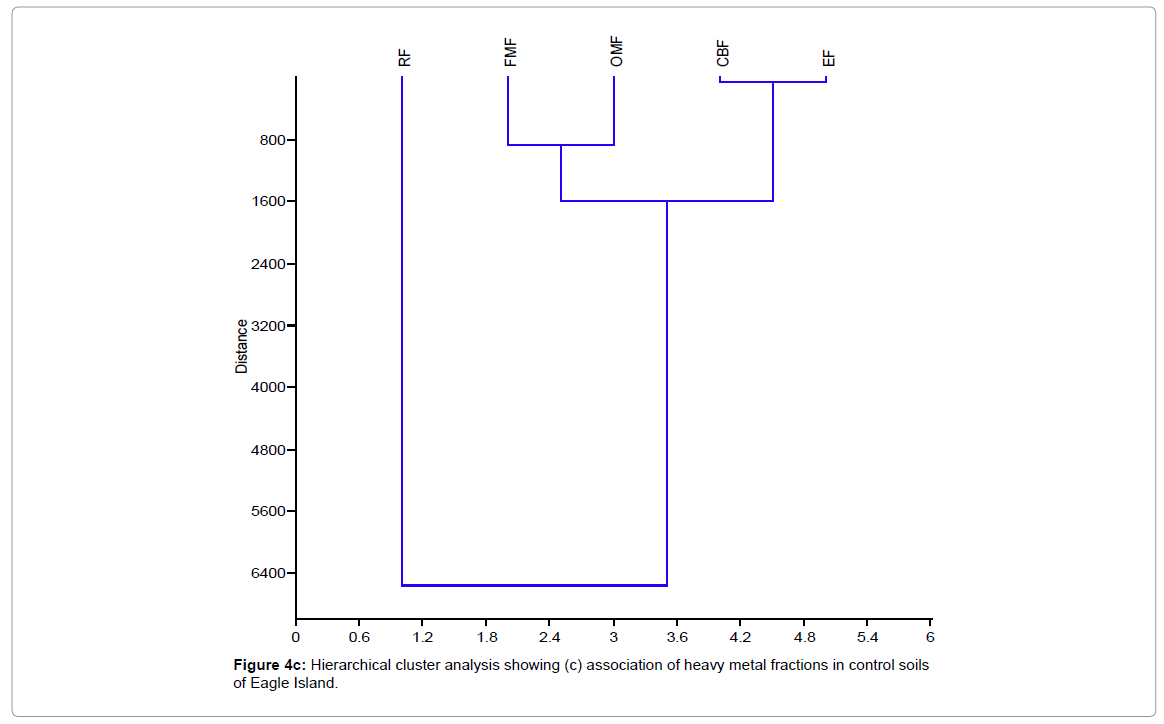
Figure 4c: Hierarchical cluster analysis showing (c) association of heavy metal fractions in control soils of Eagle Island.
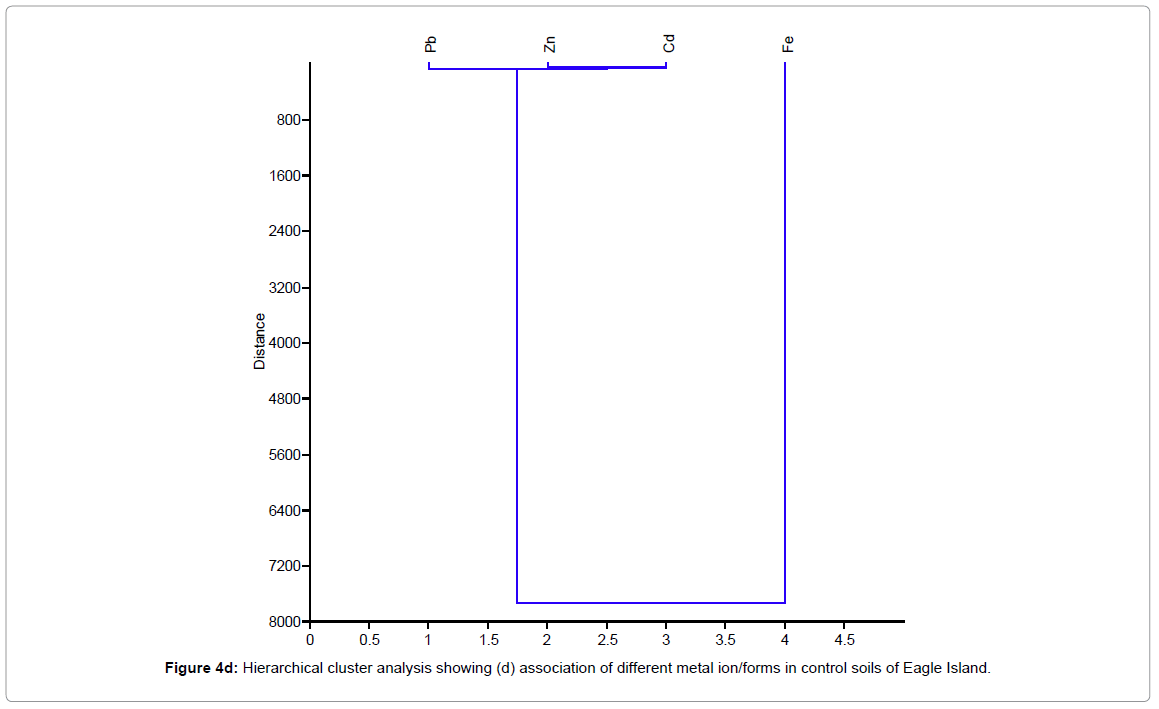
Figure 4d: Hierarchical cluster analysis showing (d) association of different metal ion/forms in control soils of Eagle Island.
Mutually dependent and independent heavy metal associations in soils of waste dumpsite
From the hierarchical cluster dendograms in Figure 4, data from soils of waste dumpsite revealed the strongest mutual dependence for Pb and Zn, while Fe and Pb reflected the highest mutual independence. In addition, residual metal forms in soils of waste dumpsite revealed strongest mutual dependence for two groups of metal species namely: (exchangeable fraction, EF and carbonate bound fraction, CBF) and (Fe- Mn oxide fraction, FMF and residual fraction, RF). On the other hand, the two most independent variables were the organic matter bound fraction (OMF) and residual fractions (RF). The mutual dependence exhibited between EF and CBF is expected as they both represent the mobile metal fractions in a soil environment. Also, the close association between FMF and RF can be attributed to their relative characteristics as poorly mobile and immobile fractions respectively, but the strong disassociation between OMF and RF may indicate that the available metals in soil are from different sources. The finding from this study is similar to Aigberua [23] where the soils enriched in organic matter bound metals were reported to have emanated predominantly from natural sources.
Mutually dependent and independent heavy metal associations in soils of control location
The hierarchical cluster dendograms in Figure 4 showed that soils of control location depicted the strongest mutual dependence for Cd and Zn, while Fe and Pb reflected the highest mutual independence. In addition, residual metal forms in soils of control location revealed strongest mutual dependence for two groups of metal species namely: (exchangeable fraction, EF and carbonate bound fraction, CBF) and (Fe-Mn oxide fraction, FMF and organic matter bound fraction, OMF). On the other hand, the two most independent variables were the exchangeable fraction (EF) and residual fractions (RF). There was similarity between soils of the control location and the waste dumpsite. Typically, the mutual dependence exhibited between EF and CBF was expected as they both represent the mobile metal fractions in a soil environment. Furthermore, the close association between FMF and OMF can be attributed to their relative characteristics as being both poorly mobile fractions. However, the strong disassociation between EF and RF may indicate that the available metals in soil are from different sources. The finding from this study is similar to Aigberua [23,24] where the soils enriched in organic matter bound metals were reported to have emanated predominantly from natural sources.
Conclusion
Generally, soils of the mangrove forest in Eagle Island were acidic and this promotes the retention of heavy metal contaminants. Even though the most prevalent metal fractions within the soil system were the poorly mobile residual fractions, the significant associations of Cd to the carbonate bound fractions (37.77%) in soils of the waste dump remains a source of environmental health concern because of its potential to become bioavailable and easily transportable via the root system of mangrove trees that serve as spawning site for many organisms consumed by human (fishes, crabs, periwinkles, etc). Its presence is also inimical to nearby farm lands growing food crops within the vicinity of the impacted area. However, Fe depicted the least environmental hazard in the soil as reflected by its position in the sequence generated for mobility factor indices: (Cd>Pb>Zn>Fe). In contrast, iron in excess amount in ground and surface water has health implication because if consumed can cause teratogenic effect, perforate the gastric mucosa and cause impotency in men. Despite the prevalence of heavy metals to the poorly transportable fractions, the significant affiliation of Cd to the readily mobile fractions of soils in the waste dump area may suggest that it emanates from the leached-out toxicants of municipal wastes. Hierarchical cluster analysis showed diverging characteristics between exchangeable and residual soil fractions across both soils of control location and the waste dumpsite, while the exchangeable and carbonate bound fractions depicted the closest association which may have resulted from their increased mobility and potential bioavailability. The disposal of waste in mangrove forest is a major environmental problem that has not been addressed, but has the potential to affect the food chain. The transfer of pollutants to human can be facilitated through bioaccumulation and biomagnification especially in acidic soils. Our result thus suggests that in other to have safe food and drinking water from the vicinity of the mangrove forests and its surrounding, refuse disposal in mangrove forest and coastal areas need to be stopped. Furthermore, there should be constant monitoring of metal levels in the study area to detect increase beyond acceptable regulatory limits and to give regular early warning to residents around the location.
Acknowledgements
We specially thank our research team comprising of students in Dr. Numbere;s Lab especially Mr. Chimezie Brown and Mr. Chamberlain Onu for assistance in sample collection. Also, to the team of Analysts from Mr. Aigberua’s Lab especially Moses Israel and Salaudeen Opeyemi who assisted with the sample preparation and analysis.
REFERENCES
- Laner D, Crest M, Scharff H, Morris JW, Barlaz MA. A review of approaches for the long-term management of municipal solid waste landfills. Waste Management. 2012;32:498-512.
- Lacerda LD, Carralho CE, Tanizaki KF, Ovalle ARC, Rezende CE. The biogeochemistry and trace metals distribution of mangrove Rhizophores. Biotropica. 1993;25:252-257.
- Numbere AO. Impact of hydrocarbon pollution on the mangrove ecosystem of the Niger River Delta, Nigeria. PhD Dissertation, Saint Louis University, Saint Louis, Missouri, USA 2014.
- Numbere AO.2018; The impact of oil and gas exploration:Invasive nypa palm species and urbanization on mangroves in the Niger River Delta, Nigeria. Coast Res Lib 25:247-266.
- Sansalone J, Ying G.2008; Partitioning and granulometric distribution of metal leachate from urban traffic dry deposition particulate matter subject to acidic rainfall and runoff retention. Water Research. 42:4146-4162.
- Zhang DQ, Tan SK, Gersberg RM. Municipal solid waste management in China:Status, problems and challenges. J Environ Management. 2010;91:1623-1633.
- Binafeigha TR, Enwin A. The State of Solid Waste Management in Port Harcourt City, Nigeria. American J Civil Eng Arch. 2017;5:160-166.
- Al-Salem SM, Lettieri P, Baeyens J. Recycling and recovery routes of plastic solid waste.PSW A review. Waste Management. 2009;29:2625-2643.
- Fernando Q. Metal Speciation in Environmental and Biological Systems. Environ Health Persp. 1995;103:13-16.
- James GK, Adegoke JO, Saba E, Nwilo P, Akinyede J. Satellite-based assessment of the extent and changes in the mangrove ecosystem of the Niger Delta. Marine Geodesy. 2007;30:249-267.
- Wang P, Numbere AO, Camilo GR. Long term changes in mangrove landscape of the Niger River Delta, Nigeria. Am J Environ Sci. 2016;12:248-259.
- Mor S, Ravindra K, Dahiya RP, Chandra A. Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environ Mon Assess. 2006;118:435-456.
- Abdullahi YA, Akunna JC, White NA, Hallett PD, Wheatley R. Investigating the effects of anaerobic and aerobic post-treatment on quality and stability of organic fraction of municipal solid waste as soil amendment. Bioresource Tech. 2008;99:8631-8636.
- Grant K, Goldizen FC, Sly PD, Brune MN, Neira M, van den Berg M, et al. Health consequences of exposure to e-waste:A systematic review. The Lancet Global Health. 2013;1:e350-e361.
- Longe EO, Balogun MR. Groundwater quality assessment near a municipal landfill, Lagos, Nigeria. Res J App Sci Eng Tech. 2010;2:39-44.
- Aigberua A, Tarawou T. Speciation and Mobility of Selected Heavy Metals in Sediments of the Nun River System, Bayelsa State, Nigeria. Environ Toxicol Stud J. 2018;2:1.
- Numbere AO. Comparison of microbial and heavy metal contents in soils and roots under mangrove forest stands with different levels of pollution in the Niger Delta, Nigeria. American J App Sci. 2017;15:132-140.
- Nóbrega GN, Ferreira TO, Romero RE, Marques AGB, Otero XL. Iron and sulfur geochemistry in semi-arid mangrove soils.Ceará, Brazil; in relation to seasonal changes and shrimp farming effluents. Environ Mon Assess. 2013;185:7393-7407.
- Osakwe SA. Chemical Speciation and Mobility of Some Heavy Metals in Soils around Automobile Waste Dumpsites in Northern Part of Niger Delta, South Central Nigeria. J Appl Sci Environ Manage. 2010;14:123-130.
- Aigberua AO, Tarawou JT, Abasi CY. Effect of oxidation-reduction fluctuations on metal mobility of speciated metals and arsenic in bottom sediments of Middleton River, Bayelsa State, Nigeria. J Appl Sci Environ Manage. 2018;22:1511-1517.
- Kabala C, Singh BR. Fractionation and Mobility of copper, lead and zinc in vicinity of copper smelter. J Environ Qual. 2001;30:485-492.
- Osakwe JO, Adowei P, Horsfall MJ. Evaluation of Heavy Metal Species in Bottom Sediments from Imo River System, Southeastern Nigeria. Res J Chem Sci. 2014;4:23-30.
- Aigberua AO. Soil organic matter:application for heavy metal source diagnostics in sediments of the Middleton River, Bayelsa State, Nigeria. MOJ Toxicol. 2018;4:410-415.
- Aigberua AO. Effects of Spatial, temporal and pH changes on fractionated heavy metals in sediments of the Middleton River, Bayelsa State, Nigeria. MOJ Toxicol. 2018;4:424-430.
Citation: Aigberua A, Numbere AO (2019) Assessment of Dumpsite Soils inMangrove Forest at Eagle Island, Nigeria: It’s Effect on Potential Bioavailability of Heavy Metals in the Environment. J Pet Environ Biotechnol 10: 390. doi: 10.35248/2157-7463.19.10.390
Copyright: © 2019 Aigber A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.