Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2021) Volume 0, Issue 0
Assessment of Bioequivalence Using Urinary Excretion Data
Eva Troja2* and Leonard Deda12Department of Medicine, University of Health and Medical Sciences, Tirana, Albania
Received: 13-Aug-2021 Published: 06-Sep-2021, DOI: 10.35248/0975-0851.21.s4.005
Abstract
A rapid method for assessing the bioavailability of the drug is the use of urinary excretion data. This method is based on the principle that the urinary excretion rate of unchanged drug is directly proportional to the plasma concentration of the drug. So bioavailability can be calculated as the ratio of the total amount of unchanged drug in urine after administration of test (T) and reference (R) formulations. Urine metabolite excretion data are not used to assess bioavailability since the drug undergoes metabolism in different locations and the rate of metabolism may vary for different reasons. This method applies to drugs that are excreted unchanged in the urine, eg, some thiazide diuretics, sulfonamides, and drugs that act on the urine, such as urinary antiseptics (Nitrofurantoin and Hexamine).
Keywords
Bioavailability; Metabolism; Diuretics; Antiseptics; Drugs
Introduction
The method involve collection of urine at regular intervals for a period of time equal to seven biological half-lives, analysis of unchanged drug in the collected sample, determination the amount of drug excreted in each interval, and the cumulative amount excreted. At each sample collection, total emptying of bladder is necessary to avoid errors resulting from addition of residuals amount to the next sample. Pharmacokinetic parameters can be calculated from the cumulative amount excreted in the urine sample at a given time interval. Frequent sampling is also essential in the beginning in order to compute correctly the rate of absorption. The decline of the plasma concentration curves and the rate of urinary excretion of the drug can be mathematically described by the same equation. Thus, it is possible to assume that the parameters obtained from the urinary excretion data reflect the absorption of the drug. However, this hypothesis is valid only when the above requirements are met. Thus, pharmacokinetic parameters and consequently, bioavailability (BD) and bioequivalence (EU) can be calculated from urinary excretion data.
Literature Review
BE studies are performed using other biological fluids such as blood, plasma and serum. The assessment of plasma pharmacokinetic parameters consists of BE determination of drug products to ensure the safety, efficacy and potency of the administered drug. The method is useful when there is lake of sufficiently techniques to measure concentration of drugs in plasma with accuracy. The method is cost-effective and noninvasive and therefore better subject compliance is assured. Convenience of collecting urine samples in comparison to drawing of blood periodically [1-4]. Often, a less sensitive analytical method is required for determining urine drug concentration as compared to plasma concentration. If the urine drug concentrations are low, assaying of larger sample volumes is relatively easy. Collection of urine samples simplifies recruiting and involving volunteers in the study. Urinary pharmacokinetic data can help to evaluate the BE of the generic products when a drug can be excreted in a significant way (about 40%) and unchanged in the urine. Among other things, urinary pharmacokinetic data are successful for drugs that have no metabolites and in small doses of the drug. Moreover, the use of blood, plasma and serum is very complex during treatment phase [5-7].
First-order elemination, excretion and absorption rate constants and fraction excreted unchanged can be computed from such data. First-order metabolism or external excretion rate constant Direct measurement of bioavailabilty, both absolute and relative, is possible without the necessary of fitting the data to a mathematical model. When coupled with plasma level-time data, Journal of Bioequivalence & Bioavailability Mini Review Correspondence to: Eva Troja, Department of Pharmacy, Profarma S.H.A. Pharmaceutical Industry, Tirana, Albania, E-mail: eva_troja@yahoo.com Received: August 13, 2021; Accepted: August 27, 2021; Published: September 3, 2021 Citation: Troja E , Deda L (2021) Assessment of Bioequivalence Using Urinary Excretion Data. J Bioequiv Availab.S4: 001. Copyright: © 2021 Troja E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. J Bioequiv Availab, Vol.13 Iss.S4 No:1000001 1 can also be calculated subsequently from the difference (kE – ke =km )
Direct measurement of bioavailabilty, both absolute and relative, is possible without the necessary of fitting the data to a mathematical model. When coupled with plasma level-time data,
it can be also used to estimate renal clearance of unchanged drug according to following equation:
ClR = Total amount of drug excreted unchanged/Area under the plasma level time curve.
If Vd is known, total systemic clearance and nonrenal clearance can also be calculated.
However, we cannot compute Vd and Clt from urine data alone. Urinary excretion data is not an accurate substitute for the plasma level data. The data can be employed as a rough estimate of pharmacokinetic parameters. If the drug product provides a very slow release or if the drug has a long biological half-life, the resulting low urinary drug concentration may be too dilute to be assessed with accuracy. A significant amount of drug must be excreted unchanged in the urine (at least 10%). The analytical method must be specific for the unchanged drug and metabolites should not interfere. Volunteers are adequately informed about the details of the study and given information and a questionnaire. Water-loading should be done by taking 400 mL of water after fasting overnight, to promote diuresis and enable collection of sufficient urine samples. The study model should be standardized. Before administration of drug, bladder must be emptied completed after 1 hour from water-loading and the urine sample taken as blank. The drug should then be administered with 200 mL of water and should be followed by 200 mL given at hourly intervals for the next 4 hours. Volunteers must be instructed to completely empty their bladder while collecting urine samples. Sampling should be continued for a sufficient period of time to ensure that the area extrapolated from the last concentration time measured at infinite time should be less than 20% of the total area below the concentration-time dependence curve. During sampling, the exact time and volume of urine excreted should be noted. An individual collection period should not exceed one biological half-life of the drug and ideally should be considerably less. Urine samples must be collected for at least 7 biological halflives in order to ensure collection of more than 99% of excreted drug. Changes in urine pH and urine volume may alter the urinary excretion rate. In contrast to plasma, urine samples are analyzed during the day. A graph of the cumulative amount of drug excreted versus time intervals of urine sample collection is obtained. The cumulative amount of drug excreted (Duc) was determined by summing the amount of drug excreted at each time interval with the amount of drug excreted at previous time intervals. The total amount of drug obtained from urine after the entire excretion period is denoted by the symbol Du∞. Using the following equation, the cumulative amount of excreted drug can be calculated as:
(Duc) i=Du∞ -(1/e-kΔt] [(Duc) i+1-(Duc) i]
where (Duc)i is the cumulative amount of drug excreted at a specific time; (Du)i+1 is the cumulative amount of drug excreted at the immediately following time; k is the constant rate of elemination; and Δt is the time interval.
The elimination half-life (t1/2)β was calculated dividing 0.693 for k. When all drugs have been excreted (t=∞) the fraction of dose absorbed after a single oral dose is given by the following equation:
F=k. Du∞/ke D0
where, F is the fraction of dose absorbed; k is the constant rate for elimination; ke is the constant rate of renal excretion; Du∞ is the total amount of drug recovered after excretion and D0 is the drug dose.
However, since elimination of the drug is usually and totally effected by renal excretion, ke=k and F=Du∞/D0
According to the study protocol, the bioequivalence hypothesis of the formulations is accepted if the 90% confidence interval of the test geometric mean to reference ratio was included within the 80-125% acceptance interval for Duc, Du∞ and (dDu/dt) max of logarithmically transformed. An example of a bioequivalence study (Figures 1 and 2) based on urinary data showed that there were no significant differences in pharmacokinetic parameters between Glucophage and Metformine products.
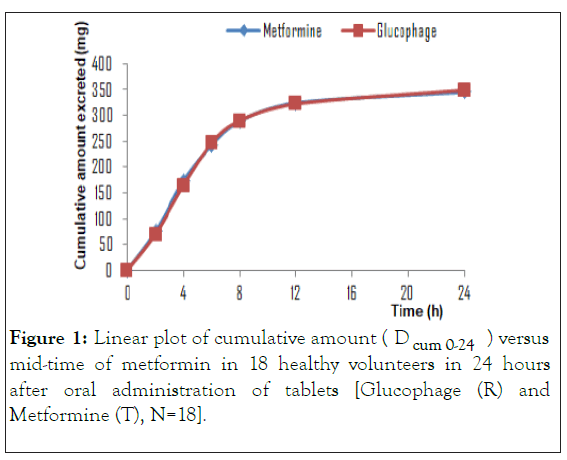
Figure 1: Linear plot of cumulative amount (Dcum 0-24) versus mid-time of metformin in 18 healthy volunteers in 24 hours after oral administration of tablets [Glucophage (R) and Metformine (T), N=18].
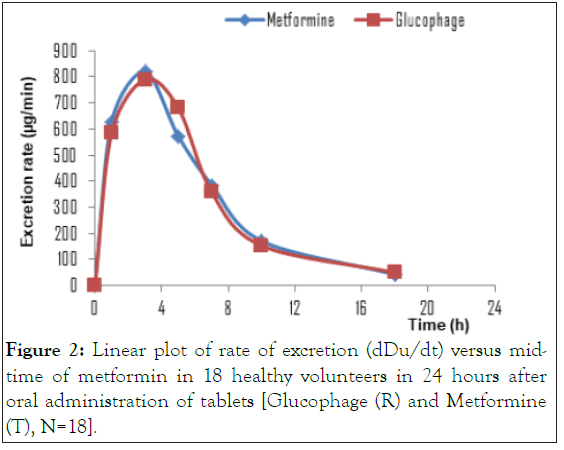
Figure 2: Linear plot of rate of excretion (dDu/dt) versus mid-time of metformin in 18 healthy volunteers in 24 hours after oral administration of tablets [Glucophage (R) and Metformine (T), N=18].
Discussion
The drug urinary excretion rate (dDu/dt) cannot be determined experimentally for any given instant. An average urinary excretion rate is then calculated for every collection period. The average values of (dDu/dt) were natural logarithmic (ln) transformed and plotted against the midpoint time of the collection period (tm). The maximum drug urinary excretion rate ((dDu/dt)max] and the middle-time (tmmax) to reach ((dDu/dt)max] were registered. The elimination constant rate (k) was determined by linear regression of the terminal phase of the logarithmically transformed (dDu/dt x tm) curve.
Conclusion
It is evident that the formulations Glucophage and Metformine exhibit a similar elimination rate, which indicates a similarity in their bioavailability. Based on this study, some drugs like metformin can be evaluated in order to predict absorption through urinary excretion data. Due to high invasiveness of bioequivalence/bioavailability conventional studies, the prediction of absorption can be performed using urinary excretion method. Furthermore, urinary excretion methodology represents low costs, it is less invasive method and its throughput is viable when compared to conventional method with blood samples.
REFERENCES
- Cawello W, Bökens H, Nickel B, Andreas JO, Halabi A. Tolerability, pharmacokinetics, and bioequivalence of the tablet and syrup formulations of lacosamide in plasma, saliva and urine: Saliva as a surrogate of pharmacokinetics in the central compartment. Epilepsia. 2013; 54 (1): 81-88.
- Shargel L, Yu A. Applied Biopharmaceutics and Pharmacokinetics. Appleton & Lange, Stamford, 1999.
- Otoom S, Hasan M, Najib N. Comparative bioavailability of two cefadroxil products using serum and urine data in healthy human volunteers. Physiol. 2004; 31 (7): 433-437.
- Portolés A, Prieto E, Calvo A, Laredo L, Fernández N, Vargas E. Truncated area under the urinary excretion rate curve in the evaluation of alendronate bioequivalence after a single dose in healthy volunteers. Arzneimittelforschung. 2009; 59 (8): 397-402.
- US Food and Drug Administration. CFR-Code of Federal Regulations Title 21, 2014.
- ArancÃbia A. Calidad biofarmacêutica. Estudos in vitro e in vivo. Acta Farm. Bonaer. 1991; 10 (2): 123-133.
- Eva Troja, Leonard Deda. Determination of bioequivalence of metformin tablets using urinary excretion data. The Eurasia Proceeding of Science, Technology, Engineering & Mathematics, 2017.
Citation: Troja E , Deda L (2021) Assessment of Bioequivalence Using Urinary Excretion Data. J Bioequiv Availab.S4: 443
Copyright: © 2021 Troja E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited

