Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 0, Issue 0
Arginine Vasopressin Promotes Transient Contractile Responses through Distinct Mechanisms in Rat Aorta and Mesenteric Resistant Arteries
Weizhong Zhu1*, Aihua Yang1, Yifeng Zhang1, Yuhang Wang1, Xiaojun Wang2, Hairong Bao1 and Jun Ren32Department of Pharmacy, Qinhuandao Workers’s Hospital, Qinhuangdao, China
3Department of Cardiology, Fudan University, Shanghai, China
Received: 25-Oct-2024, Manuscript No. JVMS-24-27264; Editor assigned: 28-Oct-2024, Pre QC No. JVMS-24-27264 (PQ); Reviewed: 11-Nov-2024, QC No. JVMS-24-27264; Revised: 18-Nov-2024, Manuscript No. JVMS-24-27264 (R); Published: 27-Nov-2024, DOI: 10.35248/2329-6925.24.S25.560
Abstract
Background: Neurohormone-regulated peripheral vascular resistance is considered one of the factors governing blood pressure. This study was designed to evaluate the effect of Arginine Vasopressin (AVP) on contraction of endothelium-intact or denuded rat aorta and mesenteric resistant arteries.
Method: The wire myograph technique was used to assess the contractility of the vascular smooth muscles in response to a high-K+, phenylephrine, AVP and inhibitors, etc. The time-course of agonist-evoked contraction was then recorded. The endothelium of the mesenteric resistance arteries and abdominal aorta were denuded by physical abrasion, as evidenced by acetylcholine-induced vasodilation dysfunction.
Results: The result revealed that: (1) AVP, but neither high K+ nor phenylephrine, evoked transient contraction of abdominal aorta and mesenteric resistance arteries; (2) Endothelial removal, V2 receptor antagonists, soluble Guanylate Cyclase (sGC) inhibitors, or Nitric Oxide (NO) synthase inhibitors reversed the transient contraction of mesenteric resistance arteries into sustained contraction, but not in aorta; (3) Pharmacological inhibition of G-Protein Coupled Receptor 2 (GPCR2) altered AVP-elicited temporal contractile response into a sustained contraction in denuded aortic endothelium; (4) The vasopressin receptor V1A blocker abolished AVP-induced contractile responses in both vessel preparations.
Conclusion: V2-mediated NO pathway in endothelium and the V1A-mediated GRK2 signaling pathways in smooth muscle are involved in AVP-induced transient contractions in rat mesenteric resistance and aortic vessels, respectively.
Keywords
Arginine vasopressin; Receptor subtype; Blood vessel; Transient contraction; G protein-coupled receptor kinase 2; Nitric oxide; Endothelium; Rat
Introduction
Multiple factors, such as the central nervous system, the adrenal glands, pituitary gland hormone secretion, renal function and fluid balance, etc., regulate peripheral blood pressure. Among them, Arginine Vasopressin (AVP) has profound cardiovascular effects beyond that it influences renal water metabolism. During hypovolemia, arginine vasopressin affects systemic blood pressure independent of the renin-angiotensin system and efferent sympathetic pathways. Physiologic levels of AVP produce a presser response in the basal state when vasoregulatory reflex are interrupted [1]. Although many studies implicate AVP in the pathogenesis of hypertension, but the actual mechanism remains unclear. The level of Arginine Vasopressin (AVP) in serum in patients with heart failure is increased [2]. Vasopressin antagonists in treatment of hyponatremia, hypertension and heart failure imply that AVP has multiple physiological effects such as water retention and vasoconstriction through vasopressin receptor subtypes, which belong to G-Protein Coupled Receptor (GPCR) [3-5].
Chronic stimulation of GPCR agonist resulting in the desensitization of GPCR is a common feature in failing heart and hypertension [6]. Continuous or repeated agonist stimulation of GPCR usually leads to a further reduced effect of agonist stimulation due to GPCR desensitization. At the same time, this process has been shown to protect the tissue against the adverse effects of excessive and inappropriate stimulation of GPCR signal transduction.
It is considered that AVP regulates blood pressure and sodium/ water balance through the potent vasoconstrictor action of the vascular V1A receptor and the diuretic effect of the renal V2 receptor [7]. However, recent results showed that AVP played a role in pathogenesis of certain genetic hypertension through V1A receptors, but the role of V1A in the maintenance of hypertension depends on the hypertensive model. For example, the V1A receptor antagonist is able to reduce mineralocorticoid-induced hypertension, but this is not the case in the normal blood pressure of rats, renovascular hypertension, Adrenocorticotropic Hormone (ACTH) induced or Nitric Oxide (NO) deficiency hypertension [7-11]. Despite V1A knockout mice showing low blood pressure, the V1A receptor may have an effect on the maintenance of blood pressure through multiple pathways other than vasoconstriction [12]. Therefore, the role of AVP as a maintenance hormone through the V1A receptor in the maintenance in blood pressure is still controversial. There are a variety of GPCRs including the V1A receptor in the blood vessels to mediate vasoconstriction and relaxation through multiple mechanisms [13]. The fact that short-term use of the AVP receptor blocker Tolvaptan (V2 receptor antagonist) or Conivaptan (V1A and V2 non-selective antagonist) improved hemodynamics and hyponatremia in patients with heart failure suggests differences in the mechanisms of action in patients with heart failure.
The interaction between vascular smooth muscle and endothelial cells determines the tension of the vascular wall. Arteries can be divided into elastic arteries, muscular arteries, small artery and arteriole according to the different anatomical structures and functions [14]. These four types of arteries are surrounded by three tunicas: Tunica intima, tunica media and tunica externa [15]. Among them, the tunica intima is composed of endothelial cells, connective tissue and smooth muscle cells. The tunica media mainly contains vascular smooth muscle and a small amount of extracellular matrix and the tunica externa is mainly composed of connective tissue [16]. For the cardiovascular system, in order to be able to supply blood to various parts in a timely manner while meeting the metabolic needs of different tissues and organs, the body adjusts blood pressure and controls blood flow by adjusting the contraction and relaxation of the vascular smooth muscle and changes the size of the vascular diameter. As the main performers of vasomotion, the vascular smooth muscle cells are regulated by many vasoactive substances in the vessel endothelium, such as Nitric Oxide (NO), Prostacyclin (PGI2), Endogenous Hyperpolarizing Factor (EDHF) and Endothelin (ET), pH of extracellular fluid, K+, Adenosine Triphosphate (ATP), adenosine, etc., [17]. In addition, the auto-regulation of vascular smooth muscle also plays an important role, such as in classical G-Protein Coupled Receptor (GPCR) signal transduction, when the GPCR on the surface is activated, it is often accompanied by the phosphorylation of GRK and the recruitment of β-arrestin to desensitize the GPCR by endocytosis, thereby terminating the continuous transmission of signals to protect the cells and maintaining their own homeostasis [18]. In order to adapt to the huge pressure fluctuations caused by cardiac ejection and relaxation, aorta play its role in pressure storage and stable blood transmission. Though its walls contain large number of smooth muscle cells, it does not show a strong vasoconstriction effect in the organic internal environment system. In contrast, peripheral resistance vessels represented by arterioles are a major contributor for maintaining blood pressure. The drug can cause vasoconstriction to a large extent, thereby affecting blood pressure by regulating peripheral resistance. While arginine vasopressin induces vasodilation in monkey coronary arteries in vitro, no additional information is available on the arginine vasopressin-regulating vessel tone, especially on different sizes of blood vessels [19].
In this study, the rat aorta and mesenteric resistant arteries were used to observe the characterization of contractile responses to AVP using pharmacological approach in vitro. The data should provide the basis of receptor subtypes of AVP on regulation of vascular endothelial cells and vascular smooth muscle cells.
Materials and Methods
Study design
Sprague-Dawley male rats with 250 g-300 g of weight were provided by the Animal Experiment Center of Nantong University. The experiments were approved by the Laboratory Animal Use and Management Committee of Nantong University. The incubation solutions are PSS (mM:130NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4•7H2O, 14.9 NaHCO3,5.5 Glucose, 0.026 EDTA, 1.6 CaCl2) and KPSS (mM:74.7 NaCl, 60 KCl, 1.18 KH2PO4,1.17 MgSO4•7H2O,14.9 NaHCO3,5.5 Glucose,0.026 EDTA,1.6 CaCl2), respectively, as shown in the designed experiments. Themicrovessel tension measurement system (DMT620M, Demark) was employed for recording the tension of vessel preparation.
Isometric contractility measurement of the rat mesenteric artery and rat aorta using wire myography
The wire myograph technique was used to assess the contractility of the vascular smooth muscles in response to depolarization, GPCR agonists, inhibitors and drug as described [20]. A 1.5 mm-2 mm segment of rat mesenteric resistance artery was isolated and mounted on a myograph chamber by passing two steel wires through its lumen. After equilibration and normalization steps, the vessel segment was potentiated by a high-K+ solution twice prior to the contraction assay. The time-course of agonist-evoked contraction was recorded. In addition, the vascular segments survived for at least 4 hours after mounting and maintained contractility induced by multiple stimulations with high-K+ solutions. The endothelium of the mesenteric resistance arteries and abdominal aorta were denuded by physical abrasion, as evidenced by acetylcholineinduced vasodilation dysfunction.
Statistical analysis
The average value of contraction induced by stimulation with high potassium solution was 100%. Vascular tonic responses to compounds were expressed as percentages of high potassium or their own maximal contractile responses as described in the results sections. The number of animals was ≥ 3 per group. Graph Pad Prism software was used for statistical analysis. Bonferroni’s test method was used for two-group comparison after two-way analysis of variance (Two-Way ANOVA) or one-way Analysis of Variance (ANOVA) for comparison of multiple groups. Student's t-test was used for comparison between two groups. Values of p<0.05 were considered significant.
Results
Temporal contractile response for arginine vasopressin in the endothelium-intact preparation of rat aorta and mesenteric resistant arteries
To compare the contractile responses for AVP in the two groups of blood vessels, we employed endothelium-intact abdominal aortic and mesenteric resistance arterial preparation. Following equilibration and normalization steps, the vessel segment was potentiated by a high-K+ solution prior to the contraction assay. The same blood vessel was challenged with 1 μM and 10 μM Phenylephrine (PE), 60 mM KCl or 0.1 μM arginine vasopressin sequentially. The preparation was rebalanced by washing 3 times with PSS following addition of each chemical. Percentage of maximum contraction was deemed as the response level for each stimulator. Figure 1A and 1B display the time-course of response curves for each simulator. The results suggested that AVP-evoked response was transient, while the contractile responses provoked by phenylephrine and high K+ was continuously maintained at the state of maximum contraction reaction within 20 min (P<0.01). Figure 1C and 1D presented area under the curve of time-course following administration of reagents. The result showed that the area under the curve of AVP was significantly smaller than that from the PE or K+ evoked responses in abdominal aorta (Figure 1).
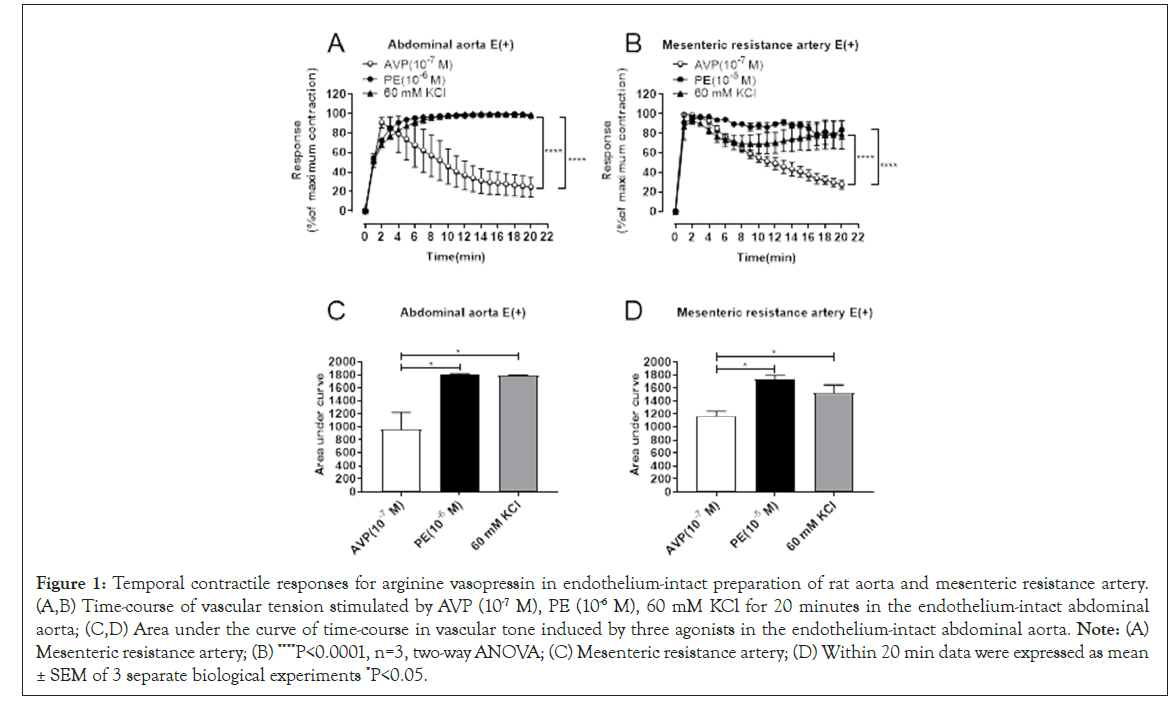
Figure 1: Temporal contractile responses for arginine vasopressin in endothelium-intact preparation of rat aorta and mesenteric resistance artery. (A,B) Time-course of vascular tension stimulated by AVP (10-7 M), PE (10-6 M), 60 mM KCl for 20 minutes in the endothelium-intact abdominal aorta; (C,D) Area under the curve of time-course in vascular tone induced by three agonists in the endothelium-intact abdominal aorta. Note: (A) Mesenteric resistance artery; (B) ****P<0.0001, n=3, two-way ANOVA; (C) Mesenteric resistance artery; (D) Within 20 min data were expressed as mean ± SEM of 3 separate biological experiments *P<0.05.
Denuded endothelium turned a transient contraction into a sustained contraction in rat mesenteric resistant arteries, but not in aorta
To discern possible involvement of endothelium in AVP-provoked transient contraction of abdominal aorta and mesenteric resistance artery, endothelium was removed by physical abrasion in mesenteric resistance artery and abdominal aorta. As shown in Figure 2A and 2B, denudation of endothelium was successfully achieved, as evidenced by loss of acetylcholine-induced vascular relaxation. However, denudation of endothelium failed to affect AVP-induced transient contraction in abdominal aorta (Figures 2C and 2D). To the contrary, endothelial removal converted AVP-induced transient contraction into a sustained contraction in mesenteric resistance artery (Figure 2E and 2F). These findings suggest that endothelium is responsible for transient contraction of mesenteric resistance artery but not rat aorta (Figure 2).
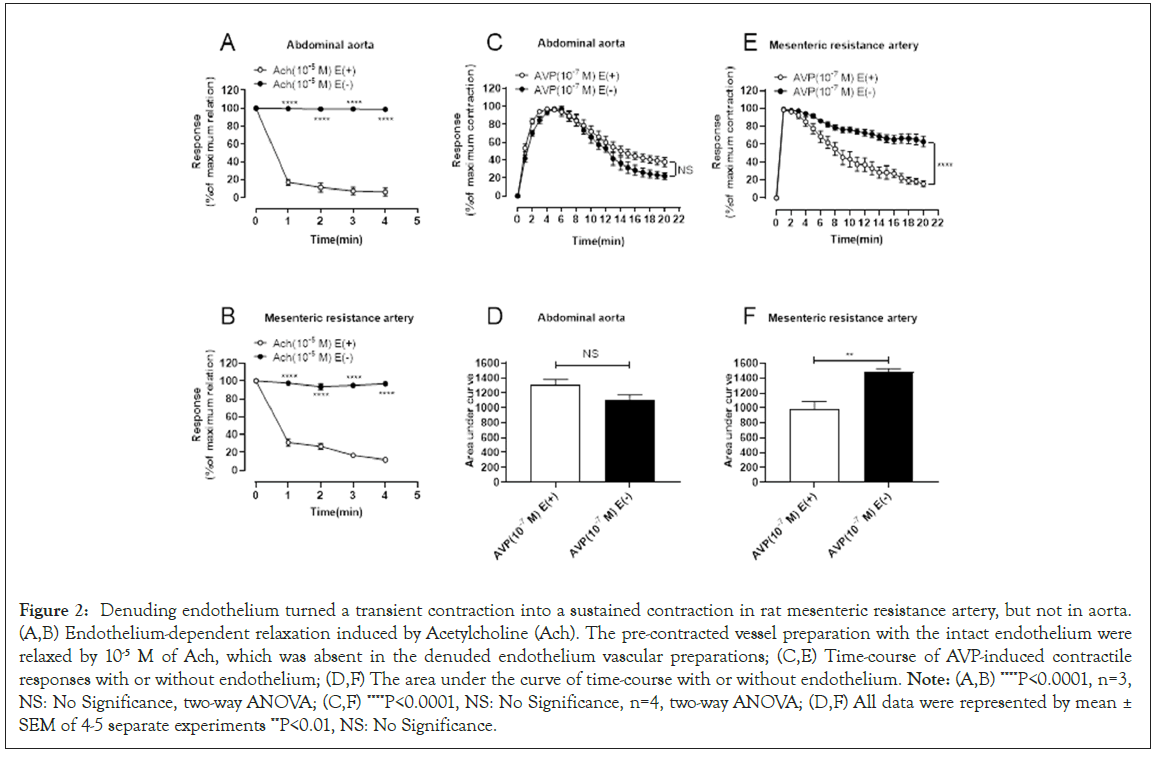
Figure 2: Denuding endothelium turned a transient contraction into a sustained contraction in rat mesenteric resistance artery, but not in aorta. (A,B) Endothelium-dependent relaxation induced by Acetylcholine (Ach). The pre-contracted vessel preparation with the intact endothelium were relaxed by 10-5 M of Ach, which was absent in the denuded endothelium vascular preparations; (C,E) Time-course of AVP-induced contractile responses with or without endothelium; (D,F) The area under the curve of time-course with or without endothelium. Note: (A,B) ****P<0.0001, n=3, NS: No Significance, two-way ANOVA; (C,F) ****P<0.0001, NS: No Significance, n=4, two-way ANOVA; (D,F) All data were represented by mean ± SEM of 4-5 separate experiments **P<0.01, NS: No Significance.
Inhibition of NOS or guanylate cyclase turned a transient contraction into a sustained contraction in rat mesenteric resistant arteries, but not in aorta
NO is a classic vasodilator factor. Both vascular endothelial cells and smooth muscle cells can use L-arginine as a precursor to generate NO under the action of nitric oxide synthase (NOS) [21]. NO acts as a small gas molecule, which can increase the content of cyclic Guanylate (cGMP) by diffusing free Guanylate Cyclase (sGC) in vascular smooth muscle cells, thereby activating cGMP-dependent Protein Kinase G (PKG) by acting on the cell membrane, reducing the influx of extracellular calcium ions; acting on the endoplasmic reticulum, reducing the calcium content in the calcium pool; activating the activity of Myosin Light Chain Phosphatase (MLCP) and inhibiting the dephosphorylation of myosin light chain. This leads to vasodilation [22,23]. In order to examine whether NO is involved in AVP-mediated transient contraction of abdominal aorta and mesenteric resistance artery, blood vessels were treated with NO Synthase (NOS) inhibitor L-Arginine Methylester (L-NAME). As shown in Figure 3A and 3B, pretreatment of endothelium-intact blood vessels with L-NAME failed to change the transient contraction into sustained contractile responses for AVP in endothelium-intact abdominal aorta, but L-NAME treatment turned the transient contraction to sustained contractile responses for AVP in endothelium-intact mesenteric resistant arteries (Figure3C and 3D). Accordingly, the inhibitor of sGC and Oxadiazolo Quinoxalin (ODQ) changed the contraction of the mesenteric resistant arteries by AVP from transient to sustained (Figures 3E and 3F). The results suggested that NOcGMP signaling was involved in contractile desensitization to AVP in mesenteric resistant arteries, but not involved in aorta (Figure 3).
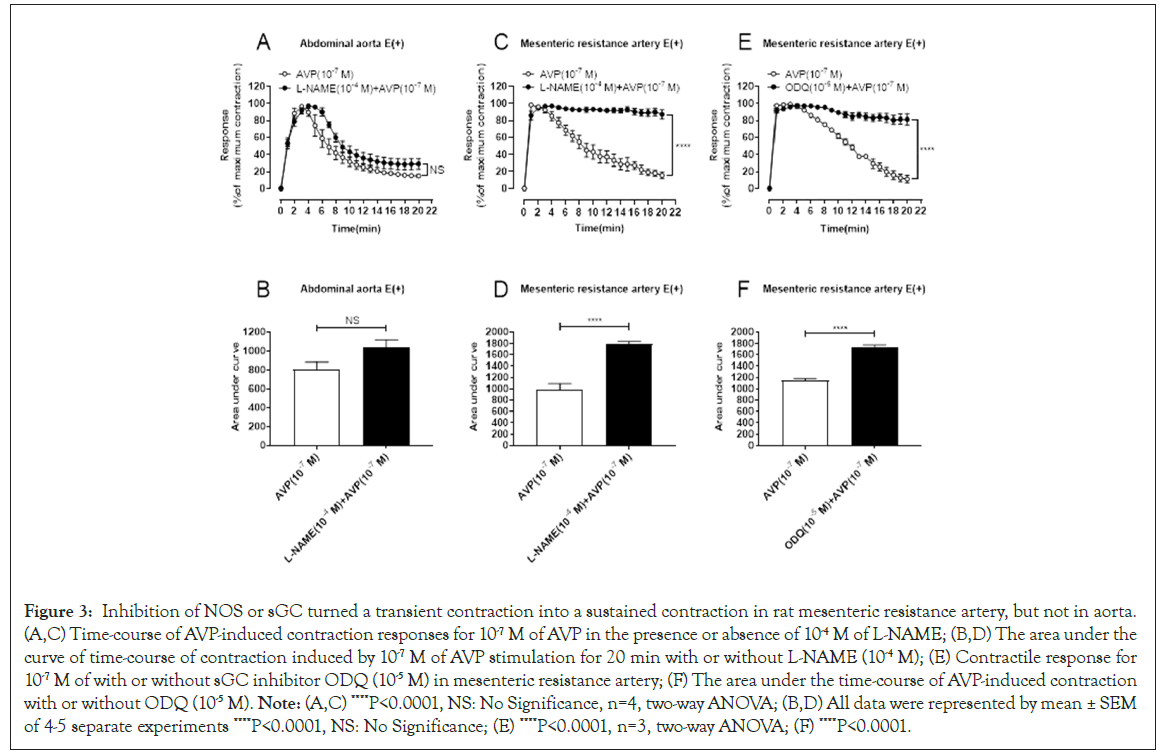
Figure 3: Inhibition of NOS or sGC turned a transient contraction into a sustained contraction in rat mesenteric resistance artery, but not in aorta. (A,C) Time-course of AVP-induced contraction responses for 10-7 M of AVP in the presence or absence of 10-4 M of L-NAME; (B,D) The area under the curve of time-course of contraction induced by 10-7 M of AVP stimulation for 20 min with or without L-NAME (10-4 M); (E) Contractile response for 10-7 M of with or without sGC inhibitor ODQ (10-5 M) in mesenteric resistance artery; (F) The area under the time-course of AVP-induced contraction with or without ODQ (10-5 M). Note: (A,C) ****P<0.0001, NS: No Significance, n=4, two-way ANOVA; (B,D) All data were represented by mean ± SEM of 4-5 separate experiments ****P<0.0001, NS: No Significance; (E) ****P<0.0001, n=3, two-way ANOVA; (F) ****P<0.0001.
Inhibition of GRK2 with pharmacological approach turned temporal contractile response for arginine vasopressin into sustained contraction in endothelium-denuded aorta, but not in de-endothelium mesenteric resistant arteries
G Protein-Coupled Receptor Kinases (GRKs) belong to the serine/ threonine protein kinase family and can be divided into G Protein- Coupled Receptor Kinases 7 subtypes (GRK1-7), which can regulate G proteins for signal transduction by phosphorylation. When GPCRs are continuously stimulated, GRKs phosphorylate GPCRs and recruit β-arrestin to internalize the receptors or stop G-protein signaling, thereby causing receptor desensitization and termination of the signaling cascade. To study whether GRK is involved in AVPmediated transient abdominal aorta contraction, the contractile responses for AVP after pretreatment of endothelium-denuded vessel preparation with the GRK2 inhibitor Paroxetine (POTH) were observed. As showed in Figure 4A and 4C, the GRK2 inhibitor POTH pretreatment kept the contractile responses for AVP more stable compared to the AVP group in endothelium-denuded aorta, suggested that GRK2 signaling is responsible for the desensitization in contractile responses for AVP stimulation. In contract, POTH pretreatment did not change the AVP-response curves (Figure 4B and 4D) in endothelium-nuded mesenteric arteries although deendothelium significantly enhanced the contractile response for AVP compared to endothelium-intact mesenteric resistance arteries (Figure 4).
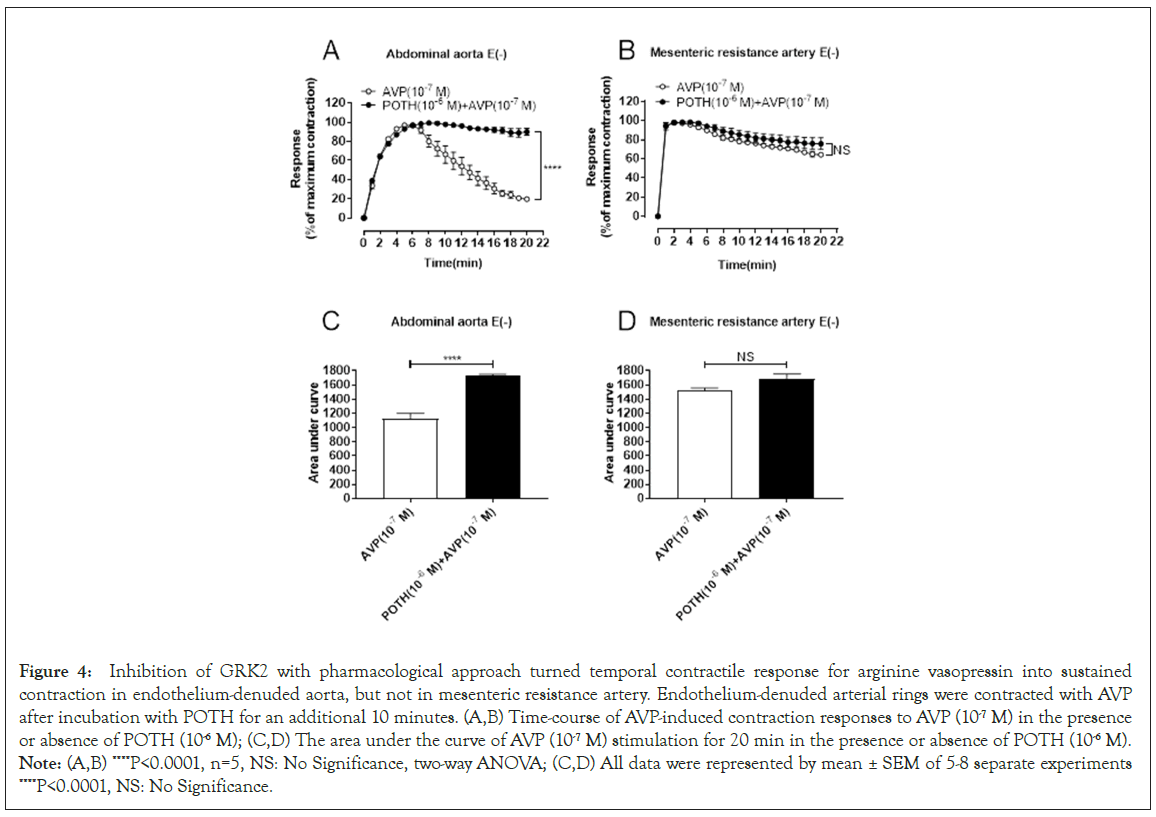
Figure 4: Inhibition of GRK2 with pharmacological approach turned temporal contractile response for arginine vasopressin into sustained contraction in endothelium-denuded aorta, but not in mesenteric resistance artery. Endothelium-denuded arterial rings were contracted with AVP after incubation with POTH for an additional 10 minutes. (A,B) Time-course of AVP-induced contraction responses to AVP (10-7 M) in the presence or absence of POTH (10-6 M); (C,D) The area under the curve of AVP (10-7 M) stimulation for 20 min in the presence or absence of POTH (10-6 M). Note: (A,B) ****P<0.0001, n=5, NS: No Significance, two-way ANOVA; (C,D) All data were represented by mean ± SEM of 5-8 separate experiments ****P<0.0001, NS: No Significance.
Arginine vasopressin receptor V2 in vessel endothelium mediated the NOS-dependent rapid desensitization in rat mesenteric resistant arteries
The V2 receptor is one of the AVP receptor subtypes, which is mainly expressed in the endothelial cells of blood vessels and the collecting ducts of renal tubules. To study whether V2 receptors are involved in AVP-induced transient contraction of the mesenteric resistance artery, the endothelium-intact vessels were pretreated with the V2 antagonist lixivaptan. Statistical results showed in Figure 5A and 5C, pretreatment of endothelium-intact aorta with 1 μM of V2 receptor antagonist were not able to reverse the transient contraction for AVP. In contract, Figure 5B and 5D displayed that, compared to the AVP-alone group, the area under the curve of the 1 μM of V2 receptor antagonist pretreatment group was significantly increased, which turned the transient contractile response for AVP to sustained contraction (Figures 5B and 5D) in endothelium-intact mesenteric arteries. It indicated that the V2 receptor subtype was involved in the transient contraction of the mesenteric resistance artery caused by AVP but could not mediate the transient contraction of the abdominal aorta (Figure 5).
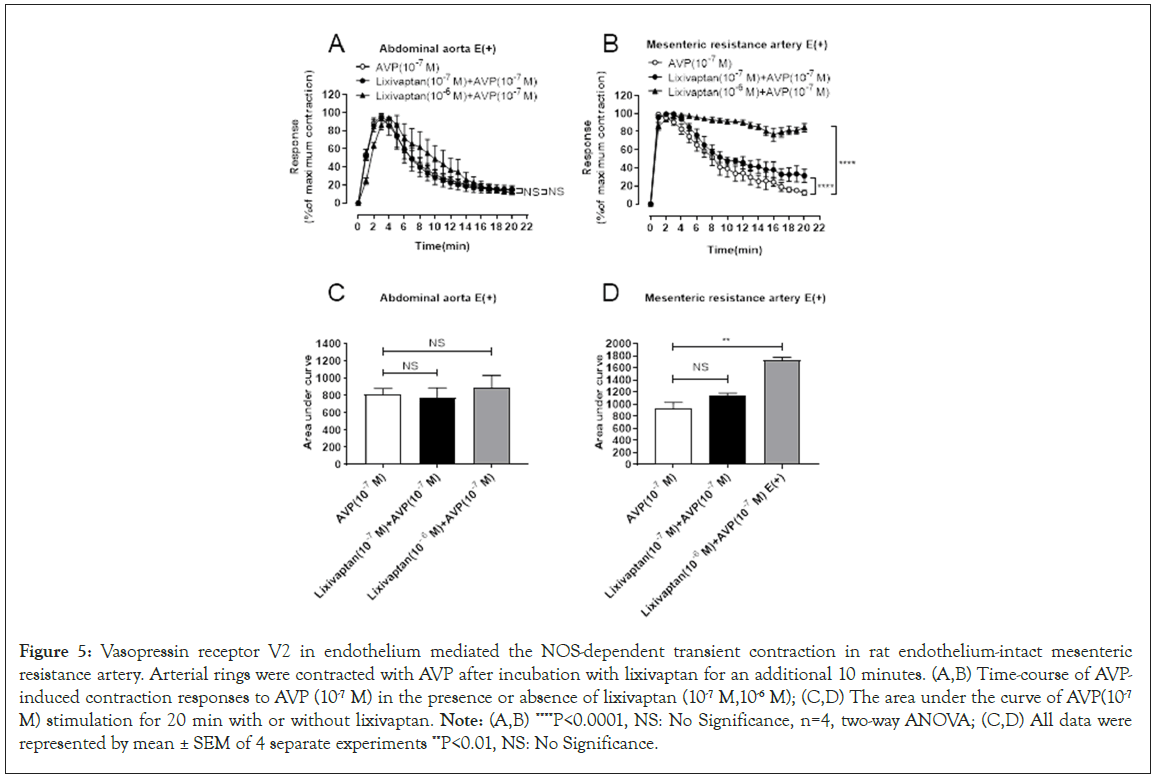
Figure 5: Vasopressin receptor V2 in endothelium mediated the NOS-dependent transient contraction in rat endothelium-intact mesenteric resistance artery. Arterial rings were contracted with AVP after incubation with lixivaptan for an additional 10 minutes. (A,B) Time-course of AVPinduced contraction responses to AVP (10-7 M) in the presence or absence of lixivaptan (10-7 M,10-6 M); (C,D) The area under the curve of AVP(10-7 M) stimulation for 20 min with or without lixivaptan. Note: (A,B) ****P<0.0001, NS: No Significance, n=4, two-way ANOVA; (C,D) All data were represented by mean ± SEM of 4 separate experiments **P<0.01, NS: No Significance.
Vasopressin receptor V1A mediated contractile responses for arginine vasopressin in endothelium-denuded vessel preparation
V1A receptors have been shown to be involved in many cellular processes, including the metabolism of glucose, lipids and proteins and vasoconstriction [24]. To confirm the role of V1A receptors in the regulation of contractile responses in aorta and mesenteric resistant arteries, the endothelium-denuded vessel was pretreated with V1A antagonist relcovaptan (SR49059). Data in Figure 6 showed that 100 nM of SR49059 pretreatment significantly inhibited the vasoconstriction response induced by AV Pin both aorta (Figure 6A and 6C) and mesenteric resistant artery (Figure 6B and 6D) compared to that inthe AVP-alone group (Figure 6).
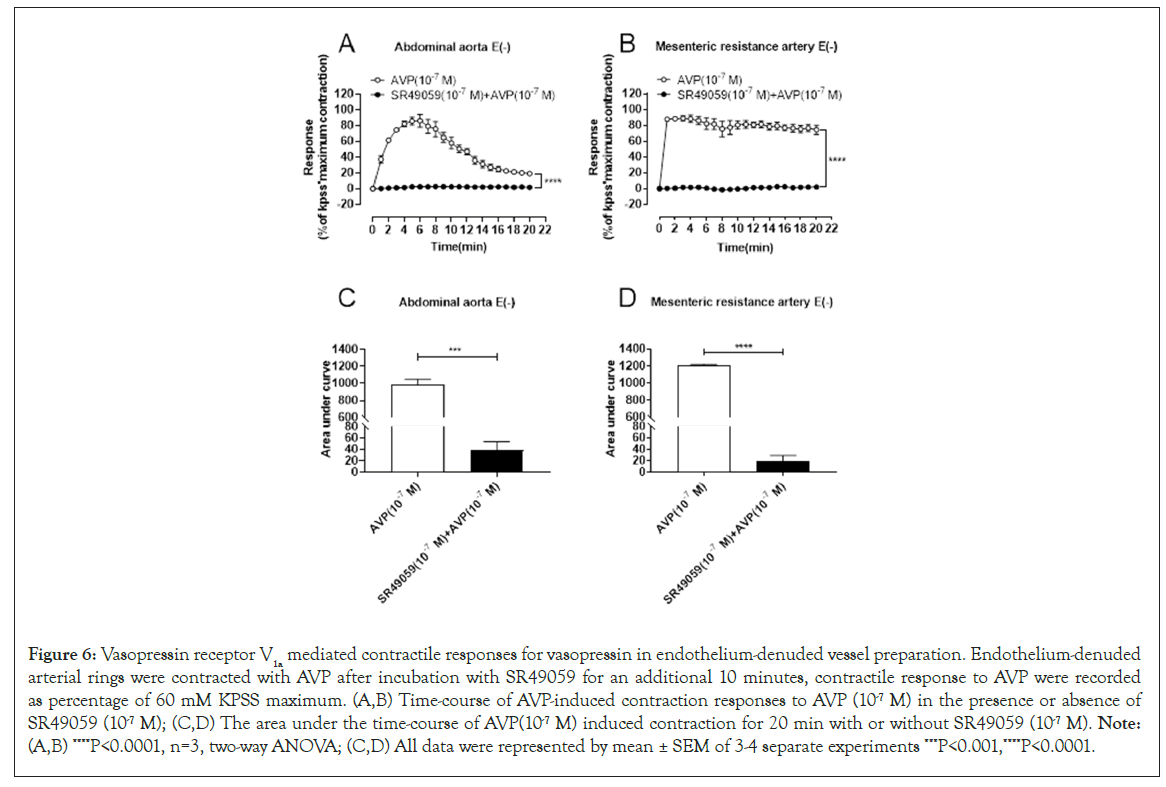
Figure 6: Vasopressin receptor V1a mediated contractile responses for vasopressin in endothelium-denuded vessel preparation. Endothelium-denuded arterial rings were contracted with AVP after incubation with SR49059 for an additional 10 minutes, contractile response to AVP were recorded as percentage of 60 mM KPSS maximum. (A,B) Time-course of AVP-induced contraction responses to AVP (10-7 M) in the presence or absence of SR49059 (10-7 M); (C,D) The area under the time-course of AVP(10-7 M) induced contraction for 20 min with or without SR49059 (10-7 M). Note: (A,B) ****P<0.0001, n=3, two-way ANOVA; (C,D) All data were represented by mean ± SEM of 3-4 separate experiments ***P<0.001,****P<0.0001.
Discussion
Hypertension, which leads to ischemic and hypertensive heart disease, stroke, peripheral atherosclerosis and renal failure, represents a multifactorial disease and is a major cause of morbidity and mortality in industrialized countries [13]. Current pharmacological therapy of essential hypertension focuses on regulating vascular resistance by inhibiting hormones such as catecholamines and angiotensin II, preventing their receptor activation. However, therapeutics resistance remains a significant issue in clinical practice. The present study found that both rat aorta and mesenteric resistant arteries showed a transient response to AVP stimulation. While the transient response for AVP in resistant arteries is regulated by the V2-NO pathway in the vascular endothelium, GRK2 mediates the transient contractile response for AVP in conduct vessels. This suggests that targeting multiple molecules could effectively regulate blood pressures in AVP signal disorder.
Endothelial cells, a type of single-layer epithelial cells that make up the luminal surface of blood vessels, are arranged longitudinally with blood flow. The endothelium can synthesize and release various substances that affect smooth muscle cell function and regulate vascular tone. To adapt to different internal and external environments and the metabolism of organs and tissues, vascular functional activity is heterogeneous, often resulting in different types of blood vessels responding differently or even oppositely to the same stimulus. As found in this study, there is a distinct difference in the vasoconstriction mechanism underlying AVP- evoked transient contraction in conducting arteries and resistant arteries. This may be due to the heterogeneity of the endothelial phenotype and vascular smooth muscle phenotype in different vascular beds, which affects the contractile phenotype of the interaction between endothelium and smooth muscle. Single-cell sequencing studies have identified six phenotypes of Endothelial Cells (EC) in vivo, quiescent EC’s maintaining homeostasis, proliferative ECs, inflammatory ECs, remodeling ECs, ECs involved in Endothelial-Mesenchymal Transition (EndMT) and ECs involved in angiogenesis [25]. These are different types of cells differentiate and perform specific functions in different tissues and organs. Similarly, for vascular smooth muscle cells, in addition to differences between contractile and synthetic types, the expression levels of contraction-mediated receptors on smooth muscle cells vary greatly among different blood vessels. Studies have shown that the primary α1-adrenoceptor subtype in the rat aorta is α1D, while in the rat renal artery, it is mainly α1A [26,27]. These factors together determine the heterogeneity of vascular structure and function, thereby adapting to the metabolic needs of different organs, which is essential for maintaining homeostasis in the body.
The synergetic action of vascular endothelium with smooth muscle evoked by AVP regulates the vascular steady state, including its tone, through subtype receptors of AVP distributed in individual vascular components. The interaction of vascular endothelium and smooth muscle, either in conduct vessels or in resistant arteries, is essential for maintaining the physiological response to AVP, directly regulating contractile response through vasopressin receptor subtypes in the blood vessel smooth muscle and endothelium.
There are three receptors in the body that can bind to AVP, namely V1A, V1B and V2 subtypes [28,29]. Among them, V1A is mainly distributed in vascular smooth muscle, adipose tissue and hepatocytes. V1B is primarily found in the central nervous system, anterior pituitary and adrenal medulla. V2 is mainly located in the basement membrane of the collecting duct of kidneys and vascular endothelial cells. In arterial tissue, the primary receptors are V1A and V2 and the transient vasoconstriction caused by AVP may be related to the receptor subtypes it binds to. The V1A subtype in vessel smooth muscle can directly modulate vessel tone, while the V2 subtype in vascular endothelium can indirectly influence it. In response to AVP, the effect of vascular endothelial cells on vascular smooth muscle cells is related to the size of vascular diameter. The V2 receptor subtype-regulated NO signaling in mesenteric vessel endothelium, but not in endothelium of conduct vessel aorta, reduces V1A-mediated the contraction. This reflects the fine regulation of arginine vasopressin on blood vessels in vascular contraction and relaxation.
Studies have shown that, in addition to activating adenyl cyclase to raise cyclic Adenosine Monophosphate (cAMP) content, activate Rap guanylate Exchange Protein directly Activated by cAMP (EPAC) factor and open cell membrane calcium channels, V2 subtypes also activate endoplasmic reticulum calcium by coupling Phospholipase C (PLC) [30]. The release of this calcium pool triggers Store-Operated Calcium Entry (SOCE), which increases intracellular calcium content. Calcium then binds to Calmodulin (CaM), activates NOS and promotes the generation of NO. The present study confirms that V2 subtypes on the endothelium of mesenteric arteries mediate NO-cGMP signaling and functionally relax the V1A-evoked contractile responses to arginine vasopressin. The PI3K/AKT/eNOS pathway could mediate such vasorelaxation [31,32]. However, present study did not find AKT signaling responsible for AVP-evoked transient contraction in endothelium- intact mesenteric resistant arteries, as the AKT inhibitor SC- 394003 was unable to abolish AVP-evoked transient contraction (Supplementary data 1).
Furthermore, the Protein Kinase C (PKC) inhibitor or stimulant PMA (Phorbol 12-myristate 13-acetate) did not turn the AVP- evoked transient to sustained contraction (Supplementary data 2). Filipin, a sterol-binding agent that disassembles endothelial non-coated plasmalemmal vesicles (caveolae), which mediate intracellular and transcellular transport of select macromolecules in the endothelium, did not alter the transient contraction (Supplementary data 3). This suggests that caveolae-mediated signaling is not associated with AVP-evoked transient contraction.
It is well known that interaction of G-Protein Coupled Receptor Kinases (GRKs) and regulator of G-Protein Signaling (RGS) proteins with activated G-Protein Coupled Receptors (GPCRs) affect the phosphorylation state of the receptors, leading to desensitization and significantly impairing signaling. Defects in GPCR regulation via these modulators have severe consequences, affecting GPCR-stimulated biological responses in pathological situations such as hypertension. These modulators fine-tune and balance the major transmitters of vessel contraction vs. dilation, making them valuable new targets for antihypertensive therapeutic strategies. Elevated levels of GRKs are associated with human hypertensive disease and are relevant modulators of blood pressure in animal models of hypertension [33,34]. Although the tone of total resistant arteries is a major contributor to maintaining blood pressure, conduit vessels also contribute through blood volume content. The present study found that V1A-evoked GRK2 is associated with the transient contraction of rat aorta and desensitization induced by AVP. It is well understood that human cells have developed multiple mechanisms to limit GPCR signaling, broadly considered as desensitization, which refers to a decrease in response to repeated or continuous stimulation. While longterm desensitization, referred to as down regulation, occurs over hours to days and involves receptor internalization into vesicles, degradation in lysosomes and decreased receptor mRNA levels through unclear mechanisms, short-term desensitization occurs over minutes and is primarily associated with β-arrestins preventing G protein interaction with a GPCR.
Phosphorylation of the receptor by GPCR kinases and the recruitment of β-arrestins are essential to both short and longterm desensitization mechanisms. In the present model, neither GRK2 nor β-arrestin 2 appears to be involved in AVP-induced desensitization in the contraction of the rat mesenteric resistance artery. Although GRK2 and arrestin 2 regulate arterial smooth muscle Purinoceptor 2 Signaling (P2Y) and are important regulators of Uridine Triphosphate (UTP) stimulated P2Y2- receptor responsiveness in resistance arteries, emphasizing their potential importance in regulating vasoconstrictor signaling pathways implicated in vascular disease [35]. Short term desensitization of the A1 adenosine receptors in DDT1MF-2 cells, has also been observed [36]. Since multiple GRK isoforms exist in smooth muscle, therapeutic approaches using the inhibition of GRK isoforms to regulate GPCRs present intriguing novel targets for the treatment of hypertension and heart failure.
Conclusion
Certain limitation exists for the present study is that the data come from vascular experiments conducted in vitro. Whether these characteristics are the same in vivo needs further investigation. The pharmacological approach could introduce non-specific effects on the targeted molecules. However, data from this study provide valuable information on the vessel-specific interaction between smooth muscle and endothelium mediated by receptor subtypes of AVP.
This study demonstrates that arginine vasopressin induces transient contractions in rat mesenteric resistance arteries and abdominal aorta. The results highlight the distinct mechanisms involved in these responses, with V2 receptor-mediated nitric oxide signaling playing a significant role in mesenteric arteries, while V1A receptor-mediated GRK2 signaling in smooth muscle contributes to the contractile response in aorta. Endothelial removal, V2 receptor antagonism and inhibition of sGC or NOS converted the transient contraction in mesenteric resistance arteries to sustained contraction. These findings suggest that different receptor and signaling pathways regulate AVP-induced contraction in distinct vascular regions.
Funding
The study was financially supported by National Natural Science Foundation of China (No. 81770400 to Weizhong Zhu).
Authors' Contributions
Conceptualization or design work conception: Aihua Yang, Weizhong Zhu, Jun Ren. Acquisition of analysis, or interpretation of data: Aihua Yang, Yifeng Zhang, Yuhang Wang, Xiaojun Wang, Hairong Bao, Weizhong Zhu. The first draft of the manuscript was written by Aihua Yang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethical Committee Consent
The experiments were approved by the Laboratory Animal Use and Management Committee of Nantong University (S20220223-033) in 28 February, 2022.
References
- Rossi NF, Schrier RW. Role of arginine vasopressin in regulation of systemic arterial pressure. Annu Rev Med. 1986;37:13-20.
[Crossref] [Google Scholar] [PubMed]
- Goldsmith SR. Vasopressin: A therapeutic target in congestive heart failure? J Card Fail. 1999;5(4):347-356.
[Crossref] [Google Scholar] [PubMed]
- Olszewski W, Głuszek J. Vasopressin antagonists in treatment of hyponatremia. Pol Arch Med Wewn. 2007;117(8):356-362.
[Google Scholar] [PubMed]
- Burrell LM, Risvanis J, Johnston CI, Naitoh M, Balding LC. Vasopressin receptor antagonism-a therapeutic option in heart failure and hypertension. Exp Physiol. 2000;85(s1):259s-265s.
[Crossref] [Google Scholar] [PubMed]
- De Vecchis R, Cantatrione C, Mazzei D. Vasopressin receptor antagonists in patients with chronic heart failure. Herz. 2017;42(5):492-497.
[Crossref] [Google Scholar] [PubMed]
- Rajagopal S, Shenoy SK. GPCR desensitization: Acute and prolonged phases. Cell Signal. 2018;41:9-16.
[Crossref] [Google Scholar] [PubMed]
- Naitoh MA, Burrell LM, Risvanis J, Aldred KL, Rockell MD, Johnston CI, et al. Modulation of genetic hypertension by short-term AVP V1A or V2 receptor antagonism in young SHR. Am J Physiol. 1997;272(2):F229-F234.
[Crossref] [Google Scholar] [PubMed]
- Burrell LM, Phillips PA, Stephenson JM, Risvanis J, Rolls KA, Johnston CI. Blood pressure-lowering effect of an orally active vasopressin V1 receptor antagonist in mineralocorticoid hypertension in the rat. Hypertension. 1994;23(6_pt_1):737-743.
[Crossref] [Google Scholar] [PubMed]
- Burrell LM, Risvanis J, Phillips PA, Naitoh M, Johnston CI. Chronic vasopressin antagonism in two-kidney, one-clip renovascular hypertension. Clin Exp Hypertens. 1997;19(5-6):981-991.
[Crossref] [Google Scholar] [PubMed]
- Fraser TB, Turner SW, Wen C, Li M, Burrell LM, Whitworth JA. Vasopressin V1A receptor antagonism does not reverse adrenocorticotrophin-induced hypertension in the rat. Clin Exp Pharmacol Physiol. 2000;27(11):866-870.
[Crossref] [Google Scholar] [PubMed]
- Loichot C, Cazaubon C, Grima M, De Jong W, Nisato D, Imbs JL, et al. Vasopressin does not effect hypertension caused by long-term nitric oxide inhibition. Hypertension. 2000;35(2):602-608.
[Crossref] [Google Scholar] [PubMed]
- Aoyagi T, Koshimizu TA, Tanoue A. Vasopressin regulation of blood pressure and volume: Findings from V1A receptor-deficient mice. Kidney Int. 2009;76(10):1035-1039.
[Crossref] [Google Scholar] [PubMed]
- Brinks HL, Eckhart AD. Regulation of GPCR signaling in hypertension. Biochim Biophys Acta. 2010;1802(12):1268-1275.
[Crossref] [Google Scholar] [PubMed]
- Zhang XT, Ruan YM, Liu YL, Yu CT. Quantitative structural study of pulmonary artery in patients with pulmonary atresia with ventricular septal defect. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Acad Med Sin. 2004;26(3):241-246.
[Google Scholar] [PubMed]
- Matsuda T, Miwa H. A hybrid vascular model biomimicking the hierarchic structure of arterial wall: Neointimal stability and neoarterial regeneration process under arterial circulation. J Thorac Cardiovasc Surg. 1995;110(4):988-997.
[Crossref] [Google Scholar] [PubMed]
- Mawad ME, Mawad JK, Cartwright J, Gokaslan Z. Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol. 1995;16(1):7-13.
[Google Scholar] [PubMed]
- Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: A role in the control of vascular tone. Trends Pharmacol Sci. 1995;16(1):23-30.
[Crossref] [Google Scholar] [PubMed]
- Li L, Homan KT, Vishnivetskiy SA, Manglik A, Tesmer JJ, Gurevich VV, et al. G protein-coupled receptor kinases of the GRK4 protein subfamily phosphorylate inactive G Protein-Coupled Receptors (GPCRs). J Biol Chem. 2015;290(17):10775-10790.
[Crossref] [Google Scholar] [PubMed]
- Okamura T, Ayajiki K, Fujioka H, Toda N. Mechanism’s underlying arginine vasopressin-induced relaxation in monkey isolated coronary arteries. J Hypertens. 1999;17(5):673-678.
[Crossref] [Google Scholar] [PubMed]
- Sun J, Yang GM, Tao T, Wei LS, Pan Y, Zhu MS. Isometric contractility measurement of the mouse mesenteric artery using wire myography. J Vis Exp. 2018;(138):58064.
[Crossref] [Google Scholar] [PubMed]
- Wu G. Functional amino acids in nutrition and health. Amino acids. 2013;45:407-411.
[Crossref] [Google Scholar] [PubMed]
- Hirst DG, Robson T. Nitric oxide physiology and pathology. Methods Mol Biol. 2011:1-3.
[Crossref] [Google Scholar] [PubMed]
- Deinum G, Stone JR, Babcock GT, Marletta MA. Binding of nitric oxide and carbon monoxide to soluble guanylate cyclase as observed with resonance Raman spectroscopy. Biochemistry. 1996;35(5):1540-1547.
[Crossref] [Google Scholar] [PubMed]
- Wasilewski MA, Myers VD, Recchia FA, Feldman AM, Tilley DG. Arginine vasopressin receptor signaling and functional outcomes in heart failure. Cell Signal. 2016;28(3):224-233.
[Crossref] [Google Scholar] [PubMed]
- Dawson A, Wang Y, Li Y, LeMaire SA, Shen YH. New technologies with increased precision improve understanding of endothelial cell heterogeneity in cardiovascular health and disease. Front Cell Dev Biol. 2021;9:679995.
[Crossref] [Google Scholar] [PubMed]
- Xin X, Yang N, Eckhart AD, Faber JE. α1D-Adrenergic receptors and mitogen-activated protein kinase mediate increased protein synthesis by arterial smooth muscle. Mol Pharmacol. 1997;51(5):764-775.
[Crossref] [Google Scholar] [PubMed]
- Armenia, Sattar MA, Abdullah NA, Khan MA, Johns EJ. α1A‐and α1D‐adrenoceptors are the major functional subtypes of renal α1‐adrenoceptors in streptozotocin-induced diabetic and normal Sprague-Dawley rats. Auton Autacoid Pharmacol. 2008;28(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Thibonnier M, Coles P, Thibonnier A, Shoham M. Molecular pharmacology and modeling of vasopressin receptors. Prog Brain Res. 2002;139:179-196.
[Crossref] [Google Scholar] [PubMed]
- Carmichael MC, Kumar R. Molecular biology of vasopressin receptors. Semin Nephrol. 1994;14(4):341-348.
[Google Scholar] [PubMed]
- O'Connor PM, Cowley Jr AW. Vasopressin-induced nitric oxide production in rat inner medullary collecting duct is dependent on V2 receptor activation of the phosphoinositide pathway. Am J Physiol Renal Physiol. 2007;293(2):F526-F532.
[Crossref] [Google Scholar] [PubMed]
- Chen JF, Liu F, Qiao MM, Shu HZ, Li XC, Peng C, et al. Vasorelaxant effect of curcubisabolanin A isolated from Curcuma longa through the PI3K/Akt/eNOS signaling pathway. J Ethnopharmacol. 2022;294:115332.
[Crossref] [Google Scholar] [PubMed]
- de Oliveira TS, de Oliveira LM, de Oliveira LP, da Costa RM, de Cassia Tostes R, de Castro Georg R, et al. Activation of PI3K/Akt pathway mediated by estrogen receptors accounts for estrone-induced vascular activation of cGMP signaling. Vascul Pharmacol. 2018;110:42-48.
[Crossref] [Google Scholar] [PubMed]
- Tutunea-Fatan E, Caetano FA, Gros R, Ferguson SS. GRK2 targeted knock-down results in spontaneous hypertension and altered vascular GPCR signaling. J Biol Chem. 2016;291(39):20822.
[Crossref] [Google Scholar] [PubMed]
- Alonazi AS, Willets JM. G protein-coupled receptor kinase 2 is essential to enable vasoconstrictor-mediated arterial smooth muscle proliferation. Cell Signal. 2021;88:110152.
[Crossref] [Google Scholar] [PubMed]
- Morris GE, Nelson CP, Everitt D, Brighton PJ, Standen NB, Challiss RJ, et al. G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling. Cardiovasc Res. 2011;89(1):193-203.
[Crossref] [Google Scholar] [PubMed]
- Nie Z, Mei Y, Ramkumar V. Short term desensitization of the A1 adenosine receptors in DDT1MF-2 cells. Mol Pharmacol. 1997;52(3):456-464.
[Crossref] [Google Scholar] [PubMed]
Citation: Zhu W, Yang A, Zhang Y, Wang Y, Wang X, Bao H, et al (2024). Arginine Vasopressin Promotes Transient Contractile Responses through Distinct Mechanisms in Rat Aorta and Mesenteric Resistant Arteries. J Vasc Surg.S25:560.
Copyright: © 2024 Zhu W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.

