Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 12, Issue 12
Application of the Lectin and Non-lectin Genes in Transgenic Crops
Suliman Khan1*, Zou Xiaobo1, Khalilur Rahman2, Rahim Dost Khan2, Muhammad Irfan3 and Mariam Jamiel32Department of Biotechnology, Abdul Wali Khan University Mardan, Mardan-23200, Pakistan
3Industrial Biotechnology Division, National Institute for Biotechnology and Genetic Engineering, Constituent College of Pakistan Institute of Engineering and Applied Sciences, PO Box 577, Jhang Road, Faisalabad, Pakistan
Received: 03-Oct-2021 Published: 30-Dec-2021
Abstract
Agricultural and horticultural crops are attacked by a number of pests, the most common of which are insect mites and nematodes, which cause damage to the plants both directly and indirectly via the fungal, bacterial, or viral infections they spread. Traditionally, agrochemicals (pesticides) were used to protect crops from pests, which had negative effects on crop yield as well as contaminating our air, affecting plant, animal, and human health. Transgenic crops that are resistant to major insect pests were one of the first achievements of plant biotechnology as a result of insects' ability to develop resistance to single insecticidal gene products. Plant with single insecticide Bacillus thuringiensis and lectin genes with resistance to major pests of rice, Maize, Tobacco, and Cotton, made up the first generation of products. The objective of this review was to discuss the application, potential, and limitation of different insect-resistant genes in transgenic crops.
Keywords
Bacillus thuringiensis; Lectin; Pest; Transgenic plant
Introduction
One of the most common and controversial biotechnology applications is transgenic crops [1,2]. To decrease dependency on insect killer sprays, researchers genetically engineered cotton and corn plants to manufacture insect killer proteins determined by genes from the communal bacterium Bacillus thuringiensis (Bt) [3]. These Bt proteins kill some of the world's most deadly insect pests while causing minimal maltreatment to other animals, as well as humans. 4.5 out of 10 Bt crops have many benefits, including decreased pesticide use, pest control, defense of advantageous natural competitors, improved yield, and higher agronomist income [4,5]. Bt crops have been cultivated on more than 420M hectares worldwide, up from 1.1M hectares in 1996 to 66 million hectares in 2011. Bt corn reported for 67% of corn planted in the US in 2012. Biotech Crops, Bt cotton reported for 79–95% of cotton planted in US, India, China and Australia between 2010 and 2012. The notable capability of insects to respond to pesticides and other control measures confirms the supposition that pest adaptation poses the greatest risk to the success of Bt crops [6-9]. Pollutants resultant from bacterium, Bt Berliner are present in all insect resistant transgenic crops currently on the market [10], but transgenic crops expressing plant-derived proteins, Snowdrop lectins Galanthus nivalis agglutinin (GNA) are currently being studied. GNA is à lectin that binds alpha-D-mannose specifically and is toxic to a variety of insect pests from various orders, including Homoptera, Coleoptera, and Lepidoptera [11]. Potato, tobacco, wheat, rice, and sugarcane are among the crop species for which transgenic lines expressing GNA have been created. According to reports, most lectin genes have different levels of gene expression to cop various abiotic pressures, such as cold, heat, drought, and salinity [12]. Both lectin genes encoded by the rice, soybean and Arabidopsis genomes were recognized and characterized [13]. However, there is no comprehensive review article that has summarized combine the application, limitation and potential of the lectins and Bt genes in different transgenic plant.
Literature Review
Lectins application in transgenic crops
Plant lectins have also been effectively used to protect crops from pest vermin [14,15]. Coleoptera, Lepidoptera, and Diptera have also been found to be toxic to lectins [15]. Used Plant lectins to fight sap-feeding insects going to the Hemiptera direction, which contains around the world's record dangerous vermin. Lectins allow nutrient interest to be blocked or midgut cells to be impaired by facilitating phagocytosis and potentially other lethal metabolites found in the hindgut. Other non-Bt genes and Plant lectins have been shown to be effective against sucking insect pests in transgenic crops (Figure1a-d). Plant-derived RNA interference (RNAi) technology has occurred as a new prospect in the fight against insects, particularly in the fight against resistance in targeted insect pests, as an alternative to conventional methods of attaining resistance, such as the use of lectins, poisonous proteins or inhibitors [16]. RNAi was first discovered in Caenorhabditis elegant [17] and has since proved to be an effective gene silencing mechanism in a number of organisms [18].
Toxicity of plant lectins towards mammals
Many plant lectins are present in a wide range of vegetables/crops (e.g. tomato, potato, pea, bean, garlic, leek, lentil, soybean, peanut, rice, corn, wheat) and fruits (e.g. banana, mulberry, breadfruit), and are consumed by humans and animals on a regular base. Since many of these plants are eaten raw, these plant lectins are considered to be non-toxic for humans and mammals in general. However, some legume lectins e.g. Concanavalin A (ConA) and Phytohaemagglutinin (PHA) are known to be toxic for mammals [19]. For example, PHA was shown to be toxic for humans especially when kidney beans were not sufficiently cooked before consumption. The acute symptoms of PHA poisoning are nausea, vomiting or diarrhea and are most likely due to the ability of PHA to bind to the epithelial cells from the digestive tract which can cause changes in cellular morphology and metabolism. It should be noted that several lectins will survive digestion by gastrointestinal enzymes. Consequently, the interaction of these plant lectins with glycoproteins in the digestive tract was reported to result in both local and systemic reactions [19]. Although toxicity was clearly shown for the broad bean (P. vulgaris) lectin considerable variation in lectin activity was observed for different beans [20]. Interestingly, the bioactivity of some plant lectins against mammalian tissues and cells could also be exploited for other applications, e.g. the use of plant lectins as potential anticancer drugs [20]. Other well-known examples of plant lectins with a severe toxicity towards mammals are ricin and abrin present in castor beans (R. communis) and the seeds of Abrus precatorius (jequirity bean), respectively [21]. However, it should be mentioned that not all ricin-B lectins are equally toxic as ricin and abrin. It has been clearly shown that ricin-B lectins from elderberry (Sambucus sp.) can be considered as virtually non-toxic compared to ricin [22]. Lectins related to the snowdrop lectin GNA have been studied in detail for their activity on insects. One of the major reasons for this large interest in GNA-related lectins is that several of these lectins are found in edible plants (e.g. leek, garlic), which will reduce the problems related to consumer acceptability whenever these lectins would be used in crop plants. A report by Fenton B, et al. [23] reported the binding of the snowdrop lectin to human white cells. However, these data are contradicted by other studies reporting very low if any mitogenic and immunogenic activity of GNA [24,25]. Since the proliferative response of the GNA-related lectin from daffodil was shown to be age-related with weak mitogenicity observed for adult human lymphocytes but more than sevenfold increased effects on lymphocytes from umbilical cord blood, it is important to check different age groups when testing the response of lectins on cells [26]. Obviously, health safety assessment for each lectin is necessary before plant lectins could be introduced into crop plants for commercial purposes. In a 90-day feeding study with rats designed to assess the safety of genetically modified rice expressing the kidney bean lectin PHA-E, clear abnormalities were observed in rats after PHA-E ingestion [27]. In contrast, a similar 90- day feeding study using transgenic rice expressing GNA revealed no adverse effects on rats after continuous dietary GNA uptake [28].
Worldwide Bt crops to pest resistance
Recent biotechnology breakthroughs have had a significant effect on agricultural crops improvement by integrating genes from different origins to establish insect pest resistance [28]. Pest vermin and pathogens, as previously said, are significant intimidations to crops, causation a 37% loss of productivity, with 13% of that loss due solely to pest vermin [29]. Since 1996, protected transgenic crops from pests, also recognized as Bt crops, have been cultivated all over the world, proving to be effective at managing pest vermin and dropping the use of toxic pesticides [30,31] (Figure 1 and Table 1). Insecticidal proteins produced by Bt bacteria are identified as Cry toxins because they form mineral presences. Based on primary sequence similarities, Cry toxins are grouped into fifty-four types (Cry1–Cry54) and various subtypes (e.g., Cry1Ba and Cry1Aa). They are particularly specialized in which they solitary affect a few insects, including lepidopteron, coleopterans, and dipterans, as well as nematodes [32,33]. Although here are other families of Cry proteins that are not 3D-Cry, the three domain (3D)-Cry family is a wide group of Cry-toxins with members that are similar in sequence and structure. Contempt the high amount of Cry toxins, only a few hundred are commercially available as sprays or in Bt crops (Cry1Ac, Cry1Ab, Cry1Aa, Cry1F, Cry1E, Cry1D, Cry1C, Cry3B, Cry3A, Cry2Ab, Cry2Aa, and Cry34/Cry35/33) (Table 2).
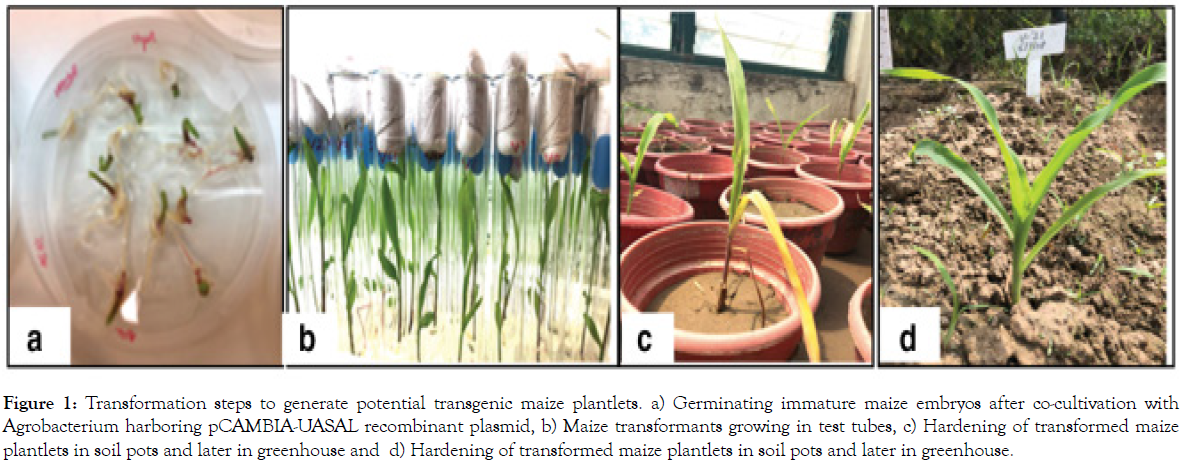
Figure 1: Transformation steps to generate potential transgenic maize plantlets. a) Germinating immature maize embryos after co-cultivation with Agrobacterium harboring pCAMBIA-UASAL recombinant plasmid, b) Maize transformants growing in test tubes, c) Hardening of transformed maize plantlets in soil pots and later in greenhouse and d) Hardening of transformed maize plantlets in soil pots and later in greenhouse.
| Crops | Gene | Application | Method | References |
|---|---|---|---|---|
| Cotton | Bt Vip3Aa | Against major insects | Agrobacterium-mediated genetic alteration | 50 |
| Bamboo | Dirigent-jacalin | Resistance to biotic and abiotic stresses |
Agrobacterium-mediated genetic alteration | 51 |
| Rice | GNA | Against the insect | Agrobacterium-mediated genetic alteration | 52 |
| Rice | ASAL | Sap-feeding insects | Agrobacterium-mediated genetic alteration | 53 |
| Tobacco | ASAL | Homopteran insects | Agrobacterium-mediated genetic alteration | 54 |
| Maize | GNA | Aphids | Agrobacterium-mediated genetic alteration | 55 |
| Wheat | Pap | Wheat Aphids | Biolistic alteration | 56 |
| Tobacco | lec-s | Pathogens and pests | Agrobacterium-mediated genetic alteration | 57 |
| Tobacco | ASAL, ASAII | Cotton leaf worm | Agrobacterium-mediated genetic alteration | 58 |
| Wheat | GNA | Aphid Sitobion avenae | Biolistic alteration | 59 |
| Potato | GNA | Aphids | Agrobacterium-derived genetic alteration | 49 |
| Maize | ASAL | Sap-feeding insects | Agrobacterium-derived genetic alteration | 60 |
| Onion | GNA | Aphid Colonization | Agrobacterium-derived genetic alteration | 61 |
| Potato | ConA | Peach-potato aphid | Agrobacterium- derived genetic alteration | 62 |
| Wheat | GNA | Grain aphid | Biolistic | 59 |
| Maize | GNA | Corn leaf aphid | Agrobacterium-derived genetic alteration | 54 |
| Chickpea | ASAL | Cowpea aphid | Agrobacterium- derived genetic alteration | 63 |
| Cotton | ACA | Cotton aphid | Agrobacterium- derived genetic alteration | 64 |
| Cotton | ASAL | Jassid and whitefly | Agrobacterium-derived genetic alteration | 65 |
| Indian mustard | ASAL | ACA (Amaranthus caudatus agglutinin) ACA-ASAL | Agrobacterium- derived genetic alteration | 66 |
| Indian mustard | (ACA-SAL) | Giving resistance against mustard aphid by reducing survival and fecundity | Agrobacterium-mediated genetic transformation of the apical meristem | 60 |
Table 1: Plant lectins have been used in a number of ways to cultivate insect-resistant crops. Pests that have been addressed as well as transition methods are explored. White backed plant hopper (WBPH), Brown plant hopper (BPH), small brown plant hopper (SBPH) and green leaf hoppers (GLH) are the four types of plant hoppers.
| S.No | Crop | Toxin | Country | Y. marketed | Dose | Insects | References |
|---|---|---|---|---|---|---|---|
| 1 | Corn | Cry1Ab | S.africa | 1998 | Low | B. fusca | 67, 68 |
| 2 | Corn | Cry1Ab | USA | 1996 | Low | H. zea | 69, 70 |
| 3 | cotton | Cry2Ab | India | 2006 | Low | P. gossypiella | 71 |
| 4 | Corn | Cry1A105 | Argentina | 2010 | Low | D. saccaharalis | 72, 73 |
| 5 | Corn | Cry1F | USA | 2003 | Low | S. prugiperda | 74, 75 |
| 6 | Corn | Cry1A.105 | USA | 2010 | Low | H. zea | 76 |
| 7 | Corn | Cry3Bb | USA | 2003 | Low | D. v. virgifera | 77, 78 |
| 8 | Corn | Cry1F | Brazil | 2009 | Low | S. prugiperda | 79, 80 |
| 9 | Cotton | Cry2Ab | USA | 2003 | Low | H. zea | 69, 81 |
| 10 | Corn | eCry3.Ab | USA | 2014 | Low | D. v. virgipera | 82, 83 |
| 11 | Cotton | Cry1Ac | USA | 1996 | Low | H. zea | 69, 70 |
| 12 | Corn | Cry1Ab | Brazil | 2008 | Low | S. prugiperda | 84 |
| 13 | Corn | Cry34/35Ab | USA | 2006 | Low | D. v. virgipera | 78, 85 |
| 14 | Cotton | Cry1Ac | India | 2002 | Low | P. gossypiella | 86, 87 |
| 15 | Corn | mCry3A | USA | 2007 | Low | D. v. virgipera | 78, 88 |
| 16 | Corn | Cry1Fa | USA | 2003 | Low | S. albicosta | 89, 90 |
| 17 | Cotton | Cry2Ab | Australia | 2004 | High | H. armigera | 91, 92 |
| 19 | Cotton | Cry1Ac | Brazil | 2013 | Low | C. includes | 93, 94 |
| 20 | Cotton | Cry1Ac | USA | 1996 | High | H. virescens | 70, 95 |
| 21 | Corn | Cr1Ab | Spain | 1998 | High | S. nonagroides | 96 |
| 22 | Cotton | Cry1Ac | China | 2000 | High | P. gossypiella | 97 |
| 23 | Corn | Cry1Ac | USA | 1999 | Low | D. grandiosella | 98, 99 |
| 24 | Corn | Cry1Ab | Spain | 1998 | Low | O. nubilalis | 99, 100 |
| 25 | Cotton | Cry1Ac | Australia | 1996 | Low | H. armigera | 101, 102 |
| 26 | Cotton | Cry1Ab | USA | 2003 | High | P. gossypiella | 103, 104 |
| 27 | Cotton | Cry1Ac | China | 2000 | High | O. nubilalis | 105, 106 |
| 28 | Cotton | Cry1Ab | Australia | 2004 | High | H. armigera | 91, 92 |
| 29 | Cotton | Cry1Ac | Mexico | 1196 | Low | H. virescens | 95 |
| 30 | Cotton | Cry1Ac | USA | 1996 | High | P. gossypiella | 104 |
| 31 | Cotton | Cry1Ac | Australia | 1996 | Low | H. punctigera | 104 |
| 32 | Corn | Cry1Fa | USA | 2003 | Low | O. nubilalis | 105, 106 |
| 33 | Corn | Vi3pA | Brazil | 2010 | High | S. frugiperda | 107 |
| 34 | Corn | Cry1Ab | USA | 1196 | Low | O. nubilali | 108, 109 |
Table 2: Practical resistant Bt crops.
Limitation and risk of insect’s resistance transgenic crops
Plant biotechnology has made significant progress in recent years, posing both prospects and threats. Transgenic and non-transgenic crops are grown in close vicinity. Insect movement from scattered arenas to transgenic crops may occur, and the increased pest weight that results can perimeter the benefits of transgenic crops. For several years, Bt toxins have been commonly used as “natural” pesticides, with no evidence of insect species developing resistance of their own [34]. Though, with the rapid rise in the prevalence of Bt toxins in the environment (due to transgenic crops), insect species could be under more strain to develop resistant biotypes. The indication on these problems is still unsatisfying, and careful monitoring is needed before large-scale transgenic crop deployment under subsistence farming conditions. One strategy for addressing these issues is to create a new group of transgenic with improved genes and to use gene groupings to slow down the emergence of resistance in insect inhabitants.
(1) Concert limits
(2) Secondary pest complications
(3) Insect compassion
(4) Progress of evolution and resistance of new biotypes
(5) Ecological effects on gene expression
(6) Gene leak into the atmosphere
(7) Possessions on non-target organisms and
(8) Bio-safety of food from transgenic crops are all issues that bound the utility of transgenic crops for pest control [34].
Plant genetic transformation Procedure, methods and their limitation
Plant genetic transformation Procedure, methods and their limitation: Indirect and direct transformation are the two most popular approaches for genetic transformation [35]. Indirect methods, which use bacteria, are discussed to as biological, whereas direct approaches, which depend on the dispersion of the cellular wall, are referred to as physical. Even though indirect approaches are still more common for plant alteration than direct approaches, physical approaches have lately become more popular. Indirect transition strategies use bacteria skilled of passing genes to higher plant species to insert plasmids, which are isolated circular DNA molecules present in bacteria that are distinct from the chromosome of bacteria into the target cell. Agrobacterium rhizogenes and Agrobacterium tumefaciens, two soil innate bacteria, are the most commonly used microorganisms [36-40] and [41,42]. A plasmid used for transformation can be somewhere between 5 and 12 kb pairs in size [43]. Plasmids contain several genes, pretend similarly to bacterial chromosomes, and are self-replicating means that they can reproduce independently inside the host. A single cell may contain up to fifty plasmids. Agrobacterium (Ag) can transmit an oncogene plasmid to its host and promote tumor growth [43-50]. This stuff (plasmid) has been used as a biotic path for genetic plant transformation, but the oncogene has been deleted (deactivated) from existing vectors, so they are no longer capable of inducing tumors. Despite its problems with regeneration of certain plants, Ag has been common in the industry [51-65] since the first active gene supplement in the 1980s [66-77]. It is broadly used for a variety of applications, but it is incomplete by the low competence of Ag transformation, mainly in monocot such as mueslis [78- 85]. Furthermore, Ag can familiarize vector sequences that aren't needed for transformation but may have unintended consequences in the plant [85-109].
Conclusion
In this study, the applications, limitations and potentials of the lectins and non lectins genes worldwide in transgenic plants were discussed. Traditional plant breeding played an important role in crop improvement in previous decades, but the introduction of genetic engineering technology revolutionized breeding methods by breaking down hybridization barriers between species and genera. The 36th anniversary of transgenic technologies for the production of genetically engineered plants is approaching. Insect pests have had a major impact on the production of farm crops all over the world. In terms of crop production and economic benefits to farmers, the commercialization of insect-resistant crops expressing Bt and lectin genes has been excellent. It's worth noting that almost all commercially available insect-resistant crops carry Bt genes. In light of the increased production of insect resistance, it is critical to look at other causes of pest resistance in addition to implementing resistance-delaying strategies.
Significance Statement
This manuscript thoroughly covers the applications and importance of the lectins, the genes associated with these proteins and as discussed as prospective biocontrol gene targets in the efforts to make transgenic plants. Different variants of the lectins are produced/synthesized by the plants in different organs most specifically seeds, roots and leaves which are primary targets of the different insects and pathogens. Recombinant DNA technology could be employed to produce transgenic plants which will not only protect plants but also be helpful to minimize the toxic effects of agrochemicals on soil and environment.
Conflicts of Interest
There are no conflicts of interest declared by the writers.
Contribution of author
Conceptualization and Writing of the original draft S.K, Revision and editing of the final version K.U.R, R.D.K, Z.Z, M.I, M.J and M.I, supervision, Z.X. All authors have read and agreed to the published version of the manuscript.
REFERENCES
- Alyokhin A. Scant evidence supports EPA's pyramided Bt corn refuge size of 5%. Nat Biotechnol. 2011;29(7):577-578.
- James C. ISAAA Briefs brief 49 Global Status of Commercialized Biotech/GM Crops. 2014.
- Sanahuja G, Banakar R, Richard M, Capell T, Christou P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol J. 2011;9(3):283-300.
- Wu K.-M, Lu Y-H, Feng H-Q, Jiang Y-Y, Zhao J-Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin–containing cotton. Science. 2008;321(5896):1676-1678
- Edgerton MD, Fridgen J, Criswll M, Dhungana P, Gocken T, et al. Transgenic insect resistance traits increase corn yield and yield stability. Nat Biotechnol. 2012;30(6):493-496.
- Pardo-Lopez L, Soberon M, Bravo A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol Rev. 2013;37(1):3-22.
- Huang F, Andow DA, Buschman LL. Success of the high-dose/refuge resistance management strategy after 15 years of Btcrop use in North America. Entomologia Experimentalis et Applicata. 2011;140(1):1-16.
- Tabashnik BE, Rensburg JV, Carrière Y. Field-evolved insect resistance to Bt crops: Definition, theory, and data. J Econ Entomol. 2009;102(6):2011-2025.
- Powell K, Gatehouse AMR, Hilder VA, Gatehouse JA, Peumans WJ. Different antimetabolic effects of related lectins towards nymphal stages of Nilaparvata lugens. Entomologia Experimentalis Et Applicata. 1995;75(1):61-65.
- Leplé JC, Pilate G, Jouanin L, Delplanque A, Augustin S. Toxicity to Chrysomela tremulae (Coleoptera: Chrysomelidae) of transgenic poplars expressing a cysteine proteinase inhibitor. Mol Breeding. 1995;1(4):319-328.
- Fitches E, Gatehouse AMR, Gatehouse JA. Effects of snowdrop lectin (GNA) delivered via artificial diet and transgenic plants on the development of tomato moth (Lacanobia oleracea) larvae in laboratory and glasshouse trials. J Insect Physiol. 1997;43(8):727-739.
- Hirano K, Teraoka T, Yamanaka H, Harashima A, Kunisaki A. Novel mannose-binding rice lectin composed of some isolectins and its relation to a stress-inducible salT gene. Plant Cell Physiol. 2000;41(3):258-267.
- Li Y, Zhang J, Hu F, Adolf A, Ackah M. Cloning and abiotic stress expression analysis of galactose-binding lectin (GBL) gene from mulberry and its prokaryotic expression in E. coli. J Hortic Sci Biotechnol. 2021;96(1):24-33.
- Goldstein IJ, Hayes CE. The lectins: Carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127-340.
- Czapla T, Lang B. Effect of plant lectins on the larval development of European corn borer (Lepidoptera: Pyralidae) and southern corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol. 1990;83(6):2480-2485.
- Price DR, Gatehouse JA. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008;26(7):393-400.
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806-811.
- Hannon GJ. RNA interference. Nature. 2002;418(6894):244-251.
- Vasconcelos IM, Oliveira JTA. Anti-nutritional properties of plant lectins. Toxicon. 2004;44(4):385-403.
- De Mejia EG, Valdez-Vega MDC, Camacho RR, Loarca-Pina G. Tannins, trypsin inhibitors and lectin cytotoxicity in tepary (Phaseolus acutifolius) and common (Phaseolus vulgaris) beans. Plant Foods Hum Nutr. 2005;60(3):137-145.
- Dickers KJ, Bradberry SM, Rice P, Griffiths GD, Vale JA. Abrin poisoning. Toxicol Rev. 2003;22(3):137-142.
- Girbes T, Ferreras JM, Arias FJ, Lglesias R, Rojo MA. Non-toxic type 2 ribosome-inactivating proteins (RIPs) from Sambucus: Occurrence, cellular and molecular activities and potential uses. Cell Mol Biol (Noisy-le-Grand). 2003;49(4):537-545.
- Fenton B, Stanley K, Fenton S, Bolton-Smith C, et al. Differential binding of the insecticidal lectin GNA to human blood cells. Lancet. 1999;354(9187):1354-1355.
- Huskens D, Vermeire K, Vandemeulebroucke E, Balzarini J, Schols D. Safety concerns for the potential use of cyanovirin-N as a microbicidal anti-HIV agent. Int J Biochem Cell Biol. 2008;40(12):2802-2814.
- François K, Balzarini J. Potential of carbohydrate-binding agents as therapeutics against enveloped viruses. Med Res Rev. 2012;32(2):349-387.
- Summers C, Forrest J, Norval M, Sharp JM. The potentially insecticidal Narcissus pseudonarcissus lectin demonstrates age-related mitogenicity. FEMS Immunol Med Microbiol. 2002;33(1):47-49.
- Poulsen M, Wilcks A, Kroghsbo S, Miller A, Frenzel T. Safety testing of GM-rice expressing PHA-E lectin using a new animal test design. Food Chem Toxicol. 2007;45(3):364-377.
- Poulsen M, Wilcks A, Kroghsbo S, Miller A, Frenzel T. A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food Chem Toxicol. 2007;45(3):350-363.
- Gatehouse AMR, Powell K, Hilder VA, Boulter D, Gatehouse JA. Potential of plant-derived genes in the genetic manipulation of crops for insect resistance. In Proceedings of the 8th International Symposium on Insect-Plant Relationships. 1992;221-234.
- Kleter GA, Bhula R, Bodnaruk K, Carazo E, Felsot AS. Altered pesticide use on transgenic crops and the associated general impact from an environmental perspective. Pest Manag Sci. 2007;63(11):1107-1115.
- Qaim M, Zilberman D. Yield effects of genetically modified crops in developing countries. Sci. 2003;299(5608):900-902.
- Crickmore N, Zeigler DR, Feitelson J, Van Rie J, Lereclus D. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62(3):807-813.
- Bravo A, Gill S, Soberon M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49(4):423-435.
- Sharma HC, Sharma KK, Crouch JH. Genetic transformation of crops for insect resistance: Potential and limitations. Crit Rev Plant Sci. 2004;23(1):47-72.
- Bajwa KS, Shahid AA, Rao AQ, Latif A, Aftab A. Expression of Calotropis procera expansin gene CpEXPA3 enhances cotton fibre strength. Aust J Crop Sci. 2013;7(2):206-212.
- Zupan J, Zambryski P, Citovsky V. The Agrobacterium DNA transfer complex. Crit Rev Plant Sci. 1997;16(3):279-295.
- Dhar MK, Kaul S, Kour J. Towards the development of better crops by genetic transformation using engineered plant chromosomes. Plant Cell Rep. 2011;30(5):799-806.
- Patnaik D, Khurana P. Wheat biotechnology: A mini review. Electron J Biotechnol. 2001;4(2):1-29.
- Christensen B, Müller R. The use of Agrobacterium rhizogenes and its rol-genes for quality improvement in ornamentals. Eur J Hortic Sci. 2009;74(6):275.
- Manimaran P, Ramkumar G, Sakthivel K, Sundaram RM, Madhav MS. Suitability of non-lethal marker and marker-free systems for development of transgenic crop plants: Present status and future prospects. Biotechnol Adv. 2011;29(6):703-714.
- Broothaerts W, Mitchell HJ, Weir B, Kaines S, Smith LMA. Gene transfer to plants by diverse species of bacteria. Nature. 2005;433(7026):629-633.
- Georgiev MI, Ludwig-Müller J, Alipieva K, Lippert A. Sonication-assisted Agrobacterium rhizogenes-mediated transformation of Verbascum xanthophoeniceum Griseb for bioactive metabolite accumulation. Plant Cell Rep. 2011;30(5):859-866.
- Hagemann R. The sexual inheritance of plant organelles, in molecular biology and biotechnology of plant organelles. Springer. 2004;93-113.
- Gelvin SB. Agrobacterium in the genomics age. Plant Physiol. 2009;150(4):1665-1676.
- Bevan MW, Flavell RB, Chilton MD. A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature. 1983;304(5922):184-187.
- Sood P, Bhattacharya A, Sood A. Problems and possibilities of monocot transformation. Biol Plant. 2011;55(1):1-15.
- Gelvin SB. Agrobacterium-mediated plant transformation: the biology behind the “Gene-Jockeying” tool. Microbiol Mol Biol Rev. 2003;67(1):16-37.
- Smith KR. Gene therapy: Theoretical and bioethical concepts. Arch Med Res. 2003;34(4):247-268.
- Bhatti MU, Riaz S, Toufiq N, Khan A, Yousaf I. The potential and efficacy of Allium sativum leaf lectin (ASAL) against sap-sucking insect pests of transgenic maize. Biologia. 2020;75:2351-2358.
- Ud Din S, Azam S, Rao AQ, Shad M, Ahmed M. Development of broad-spectrum and sustainable resistance in cotton against major insects through the combination of Bt and plant lectin genes. Plant Cell Rep. 2021;40(4):707-721.
- Nagadhara D, Ramesh S, Pasalu IC, Rao YK, Sarma NP, et al. Transgenic rice plants expressing the snowdrop lectin gene (gna) exhibit high-level resistance to the white backed plant hopper (Sogatella furcifera). Theor Appl Genet. 2004;109(7):1399-1405.
- Saha P, Majumder P, Dutta I, Ray T, Roy SC,. Transgenic rice expressing Allium sativum leaf lectin with enhanced resistance against sap-sucking insect pests. Planta. 2006;223(6):1329-1343.
- Dutta I, Saha P, Majumder P, Sarkar A, Chakraborti D. The efficacy of a novel insecticidal protein, Allium sativum leaf lectin (ASAL), against homopteran insects monitored in transgenic tobacco. Plant Biotechnol J. 2005;3(6):601-611.
- Wang Z, Zhang K, Sun X, Tang K, Zhang J. Enhancement of resistance to aphids by introducing the snowdrop lectin gene gna into maize plants. J Biosci. 2005;30(5):627-638.
- Mi X, Liu X, Yan H, Liang L, Zhou X. Expression of the Galanthus nivalis agglutinin (GNA) gene in transgenic potato plants confers resistance to aphids. C R Biol. 2017;340(1):7-12.
- Duan X, Hou Q, Liu G, Pang X, Niu Z. Expression of Pinellia pedatisecta lectin gene in transgenic wheat enhances resistance to wheat aphids. Molecules. 2018;23(4):748.
- Guo P, Wang Y, Zhou X, Xie Y, Wu H. Expression of soybean lectin in transgenic tobacco results in enhanced resistance to pathogens and pests. Plant Sci. 2013;211:17-22.
- Sadeghi A, Smagghe G, Broeders S, Hernalsteens JP, Greve HD. Ectopically expressed leaf and bulb lectins from garlic (Allium sativum L.) protect transgenic tobacco plants against cotton leaf worm (Spodoptera littoralis). Transgenic Res. 2008;17(1):9.
- Stoger E, Williams S, Christou P, Down ER, Gatehouse JA. Expression of the insecticidal lectin from snowdrop (Galanthus nivalis agglutinin; GNA) in transgenic wheat plants: effects on predation by the grain aphid Sitobion avenae. Mol Breeding. 1999;5(1):65-73.
- Hossain MA, Maiti KM, Basu A, Sen S, Gosh KA. Transgenic expression of onion leaf lectin gene in Indian mustard offers protection against aphid colonization. Crop Sci. 2006;46(5):2022-2032.
- Lacroix B, Citovsky V. The roles of bacterial and host plant factors in agrobacterium-mediated genetic transformation. Int J Dev Biol. 2013;57(6-7-8):467-481.
- Gatehouse AM, Davison MG, Stewart J, Gatehouse NL, Kumar A. Concanavalin A inhibits development of tomato moth (Lacanobia oleracea) and peach-potato aphid (Myzus persicae) when expressed in transgenic potato plants. MoleBreeding. 1999;5(2):153-165.
- Chakraborti D, Sarkar A, Ali Mondal H, Das S. Tissue specific expression of potent insecticidal, Allium sativum leaf agglutinin (ASAL) in important pulse crop, chickpea (Cicer arietinum L.) to resist the phloem feeding Aphis craccivora. Transgenic Res. 2009;18(4):529-544.
- Wu J, Luo X, Guo J, Xiao J, Tian Y. Transgenic cotton, expressing Amaranthus caudatus agglutinin, confers enhanced resistance to aphids. Plant Breeding. 2006;125(4):390-394.
- Vajhala, CS, Sadumpati VK, Nunna HR, Puligundla SK, Vudem DR. Development of transgenic cotton lines expressing Allium sativum agglutinin (ASAL) for enhanced resistance against major sap-sucking pests. PLoS One. 2013;8(9): e72542.
- Dutta I, Majumder P, Saha P, Ray K, Das S. Constitutive and phloem specific expression of Allium sativum leaf agglutinin (ASAL) to engineer aphid (Lipaphis erysimi) resistance in transgenic Indian mustard (Brassicajuncea). Plant Sci. 2005;169(6):996-1007.
- Van Rensburg JBJ. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S Afr J Plant Soil. 2007;24(3):147-151.
- Van Rensburg J. Evaluation of Bt.-transgenic maize for resistance to the stem borers Busseola fusca (Fuller) and Chilo partellus (Swinhoe) in South Africa. S Afr J Plant Soil. 1999;16(1):38-43.
- Tabashnik BE, Brévault T, Carrière Y. Insect resistance to Bt crops: lessons from the first billion acres. Nature Biotechnol.2013;31(6):510-521.
- Ali M, Luttrell RG, S. Young III. Susceptibilities of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) populations to Cry1Ac insecticidal protein. J Econ Entomol. 2006;99(1):164-175.
- Kranthi K. Pink bollworm strikes Bt-cotton. Cotton Statistics & News. 2015;35(1):6.
- Murúa MG. Situation and perspectives of insect resistance management (IRM) in Bt crops in Argentina. In 2016 International Congress of Entomology. 2016. ESA.
- Blanco CA, Chiaravalle W, Dalla-Rizza M, Farias JR, Gastaminza G. Current situation of pests targeted by Bt crops in Latin America. Curr Opin Insect Sci. 2016;15:131-138.
- Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol. 2010;103(4):1031-1038.
- Huang F, Qureshi JA, Jr RLM, Reisig DD, Head GP. Cry1F resistance in fall armyworm Spodoptera frugiperda: Single gene vs. pyramided Btmaize. PloS One. 2014;9(11):e112958.
- Dively GP, Venugopal PD, Finkenbinder C. Field-evolved resistance in corn earworm to Cry proteins expressed by transgenic sweet corn. PloS One. 2016;11(12):e0169115.
- Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW. Field-evolved resistance to Bt maize by western corn rootworm. PloS One. 2011;6(7):e22629.
- Andow DA, Pueppke SG, Schaafsma AW, Gassmann AJ, Sappington TW. Early detection and mitigation of resistance to Btmaize by western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol. 2016;109(1):1-12.
- Farias JR, Andow DA, Horikoshi RJ, Sorgatto RJ, Fresia P. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop protection.2014;64:150-158.
- Farias JR, Andow DA, Horikoshi RJ, Sorgatto RJ, Santos ACD. Dominance of Cry1F resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) on TC1507 Bt maize in Brazil. Pest Manag Sci. 2016;72(5):974-979.
- Ali M, Luttrell RG. Susceptibility of bollworm and tobacco budworm (Lepidoptera: Noctuidae) to Cry2Ab2 insecticidal protein. J Econ Entomol. 2007;100(3):921-931.
- Zukoff SN, Ostlie KR, Potter B, Meihls LN, Zukoffet AL, et al. Multiple assays indicate varying levels of cross resistance in Cry3Bb1-selected field populations of the western corn rootworm to mCry3A, eCry3. 1Ab, and Cry34/35Ab1. J Econ Entomol. 2016;109(3):1387-1398.
- Jakka SR, Shrestha RB, Gassmann AJ. Broad-spectrum resistance to Bacillus thuringiensis toxins by western corn rootworm (Diabrotica virgifera virgifera). Sci Rep. 2016;6(1):1-9.
- Omoto C, Bernardi O, Salmeron E, Sorgatto RJ, Dourado PM. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag Sci. 2016;72(9):1727-1736.
- Ludwick D, Meihls LN, Ostlie KR, Potter BD, French L, et al. Minnesota field population of western corn rootworm (Coleoptera: Chrysomelidae) shows incomplete resistance to Cry34Ab1/Cry35Ab1 and Cry3Bb1. J Appl Entomol. 2017;141(1-2):28-40.
- Mohan KS, Ravi KC, Suresh PJ, Sumerford D, Head GP. Field resistance to the Bacillus thuringiensis protein Cry1Ac expressed in Bollgard (®) hybrid cotton in pink bollworm, Pectinophora gossypiella (Saunders), populations in India. Pest Manag Sci.2016;72(4):738-746.
- Nair R, Kamath SP, Mohan KS, Head G, Sumerford DV. Inheritance of field‐relevant resistance to the Bacillus thuringiensis protein Cry1Ac in Pectinophora gossypiella (Lepidoptera: Gelechiidae) collected from India. Pest Manag sci. 2016;72(3):558-565.
- Gassmann AJ, Shrestha RB, Kropf AL, Clair CRS, Brenizer BD. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Pest Manag Sci.2 014;111(14):5141-5146.
- Ostrem JS, Pan Z, Flexner JL, Owens E, Binning R, et al. Monitoring susceptibility of western bean cutworm (Lepidoptera: Noctuidae) field populations to Bacillus thuringiensis Cry1F protein. J Econ Entomol. 2016;109(2): 847-853.
- Eichenseer H, Strohbehn R, Burks JC. Frequency and severity of western bean cutworm (Lepidoptera: Noctuidae) ear damage in transgenic corn hybrids expressing different Bacillus thuringiensis cry toxins. J Econ Entomol.2014;101(2):555-563.
- Walsh TK, Downes SJ, Gascoyne J, James W, Parker T. Dual Cry2Ab and Vip3A resistant strains of Helicoverpa armigera and Helicoverpa punctigera (Lepidoptera: Noctuidae); testing linkage between loci and monitoring of allele frequencies. J Econ Entomol. 2014;107(4):1610-1617.
- Downes S, Walsh T, Tay WT. Bt resistance in Australian insect pest species. Curr Opin Insect Sci. 2016;15:78-83.
- Yano SA, Specht A, Moscardi F, Carvalho RA, Dourado PM, et al. High susceptibility and low resistance allele frequency of Chrysodeixis includens (Lepidoptera: Noctuidae) field populations to Cry1Ac in Brazil. Pest Manag Sci. 2016;72(8):1578-1584.
- Tabashnik BE, Carrière Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol. 2017;35(10):926.
- Blanco CA, Andow DA, Abel CA, Sumerford DV, Hernandez G. Bacillus thuringiensis Cry1Ac resistance frequency in tobacco budworm (Lepidoptera: Noctuidae). J Econ Entomol. 2009;102(1):381-387.
- Castañera P, Farinós GP, Ortego F, Andow DA. Sixteen years of Bt maize in the EU hotspot: why has resistance not evolved? PLoS One.2016;11(5):e0154200.
- Wan P, Xu D, Cong X, Jiang Y, Huang Y, et al. Hybridizing transgenic Bt cotton with non-Bt cotton counters resistance in pink bollworm. Proc Natl Acad Sci. 2017;114(21):5413-5418.
- Huang, F, Leonard BR, Cook RD, Lee RD, Andow AD,. Frequency of alleles conferring resistance to Bacillus thuringiensis maize in Louisiana populations of the southwestern corn borer. Entomol Exp Appl. 2007;122(1):53-58.
- Organisms EPoGM. Scientific Opinion on the annual post-market environmental monitoring (PMEM) report from Monsanto Europe SA on the cultivation of genetically modified maize MON 810in2013.EFSA J. 2015;13(3):4039.
- Organisms EPoGM. Scientific Opinion on the annual Post-market Environmental monitoring (PMEM) report from Monsanto Europe SA on the cultivation of genetically modified maize MON 810 in 2010. EFSA J. 2012;10(4):2610.
- Downes S, Bird L. End of season resistance monitoring report and insecticide testing. 2015.
- Bird LJ, Akhurst RJ. Fitness of Cry1A-resistant and-susceptible Helicoverpa armigera (Lepidoptera: Noctuidae) on transgenic cotton with reduced levels of Cry1Ac. J Econ Entomol. 2005;98(4):1311-1319.
- Fabrick JA, Unnithan GC, Yelich AJ, Gain BD, Masson L. Multi-toxin resistance enables pink bollworm survival on pyramided Bt cotton. Sci Rep. 2015;5:16554.
- Tabashnik BE, Sisterson MS, Ellsworth PC, Dennehy TJ, Antilla L. Suppressing resistance to Bt cotton with sterile insect releases. Nat biotechnol. 2010;28(12):1304.
- Siegfried BD, Rangasamy M, Wang H, Spencer T, Haridas CV. Estimating the frequency of Cry1F resistance in field populations of the European corn borer (Lepidoptera: Crambidae). Pest Manag Sci. 2014;70(5):725-733.
- Pereira EJG, Storer NP, Siegfried BD. Inheritance of Cry1F resistance in laboratory-selected European corn borer and its survival on transgenic corn expressing the Cry1F toxin. Bull Entomol Res. 2008;98(6):621-629.
- Bernardi O. Frequency of resistance to Vip3Aa20 toxin from Bacillus thuringiensis in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. Crop Prot. 2015;76:7-14.
- Crespo AL, Spencer TA, Alves AP, Hellmich RL, Blankenship EE. On-plant survival and inheritance of resistance to Cry1Ab toxin from Bacillus thuringiensis in a field-derived strain of European corn borer, Ostrinia nubilalis. Pest Manag Sci. 2009;65(10):1071-1081.
- Siegfried BD, Hellmich RL. Understanding successful resistance management: The European corn borer and Btcorn in the United States. GM Crops Food. 2012;3(3):184-193.
Citation: Khan S, Xiaobo Z, Rahman K, Dost Khan R, Irfan M, Jamiel M, et al. (2021) Application of the Lectin and Non-lectin Genes in Transgenic Crops. J Plant Pathol Microbiol. 12:590.
Copyright: © 2021 Khan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.