Indexed In
- Open J Gate
- JournalTOCs
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- Publons
- Geneva Foundation for Medical Education and Research
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2023) Volume 11, Issue 4
Anti-germ and Hygiene Finishing of Textiles for Human Health and Safety
Shriyasha Tari and Ashok Athalye*Received: 01-Aug-2023, Manuscript No. MCA-23-22436; Editor assigned: 03-Aug-2023, Pre QC No. MCA-23-22436 (PQ); Reviewed: 18-Aug-2023, QC No. MCA-23-22436; Revised: 28-Aug-2023, Manuscript No. MCA-23-22436 (R); Published: 05-Sep-2023, DOI: 10.35248/2329-6798.23.11.425
Abstract
The recent COVID-19 pandemic has explained the need for antigerm finish for textiles used by frontline healthcare and hospitality workers. Apart from the hospitality industry and hygiene wear, other textile applications have essential anti-microbial, anti-bacterial, antiviral or anti-fungal finishes. Military uniforms worn by soldiers in extreme conditions, intimate clothing like undergarments, socks, sportswear used by athletes and players, surgical wound dressings used in medical textiles, filters used in air and water purification systems, home furnishing fabrics are other application areas for anti-germ finishes.
Keywords
Antimicrobial effect, Eco toxicity, Health and safety, Hygiene finish, Protective textiles
Introduction
Finishing is the last step in the textile processing segment. Textile finishing is broadly categorized into protective and perceptive finishing based on the desired end use and customer requirement. Softness, absorbency, and moisture management (breathability) are needed in fabric for aesthetic reasons, as clothes are worn in intimate contact with the skin. Such effects are imparted in fabrics by means of perceptive finishes. The other class of finishes i.e., protective finishes, provide resistance from external natural or synthetic agents. For example, water-repellent finishes are used in making umbrellas, tents etc. Other examples of protective finishes used for textiles are oil and stain repellants, flame retardants, and anti-germ finishes.
In this review, ‘Antigerm’ is a broad term that consists of antimicrobial, anti-bacterial, anti-viral, insect-repellant finishes used for textiles. Textiles serve as a good host for such agents like bacteria, viruses, and microbes as the surface area, pH, humidity of fabric, and accumulated sweat are favorable conditions for the growth of such pathogens. Anti-germ finishes resist such organisms and protect the wearer from harmful infections.
Each antigerm finish has a specific mode of action against pathogens. For instance, there are two variants of antimicrobials depending on the action mechanism. One is Bacteriostatic which inhibits the growth of bacteria on the surface of textiles, while the other one is bactericidal, which kills the bacteria. The synthesis, mechanism and application of such anti-germ textile finishes have been an area of research interest for many decades. There has been a lot of academic and industrial research on developing and elucidating anti-germ and hygiene finish chemistries. However, the environmental and safety concerns, and risks associated with such finishing chemistry, specifically with respect to fabrics, are not well elaborated in the literature. This review emphasizes the evolution of anti-germ finishes, limitations in existing chemistries of antigerm finishes for textiles, and the toxicity and hazards posed to the wearer and the ecosystem on the disposal of such textiles.
Literature Review
Anti-germ finishes and their applications
Natural fibres are more prone to microbial action because of their moisture retention, and hydrophilicity. Synthetic fibers portray strong resistance to microbes. However, manmade fibers containing cellulose, like viscose rayon, cellulose acetate, are also susceptible to microbial action. The most popularly used commercial chemistries of anti-germ finishes are enlisted in Table 1, based on their origin and synthesis routes. These chemistries are incorporated into fabrics to create a protective layer between the fabric and the wearer’s interface. Depending on their chemistry, they work against a range of biological agents like bacteria, viruses, microbes, fungi, moulds, etc.
| Anti-germ textile finishes | Chemistry |
|---|---|
| Inorganic chemistry derivatives | Metals and metal salts, metallic nanoparticles |
| Organic chemistry derivatives | Triclosan, phmb, quaternary ammonium compounds, n-halamines, and conjugated polymers like polypyrrole |
| Products from natural origin | Natural plant extracts, chitosan, essential oil, cyclodextrin encapsulated products |
Table 1: Anti-germ textile finishes
These chemistries are widely used in finishing textiles for the past few decades. Most fabrics are treated with these finishing agents using conventional application techniques like exhaust-batch, and continuous (Pad-Dry-Cure). In the case of synthetic fibers. It is possible to incorporate these finishes in the polymer melt itself at the time of extrusion. Electrospinning can also be used to produce intrinsically antimicrobial fibers. Micro-encapsulation technique is also used to get such functional finishes on textiles. Modern surface modification techniques like UV, Ultrasound and plasma have also been explored for getting antimicrobial effects on textile substrates (Table 1).
Apart from spreading diseases and infections through textiles contaminated with germs, there are other undesired ill effects. Fabrics and garments infested with germs result in foul odour, and show shade change and loss in tensile strength. Therefore, antigerm finishes play a crucial role in shielding the wearer from germs, and preventing the fabric from undesired effects while acting as a protective barrier between fabric and the biological contaminants. Owing to these benefits, the need for anti-bacterial, anti-fungal, and anti-microbial finishes has grown exponentially in the last two decades. The global anti-microbial textile market will reach $12,313 by 2024 [1]. The rising need for hygienic wear and exorbitant use of antigerm finishes portray a serious threat to the environment and ecosystem. The serious concerns associated with these finishing agents are enlisted with respect to the chemistries of such antigerm agents in the following sections of this review.
The hygiene products like personal protective textiles, facemasks, curtains, wipes, and bandages are treated with anti-microbial and hygiene finishes. Most of these products are meant for single use and are disposable. If this waste is not well segregated, few of them are incinerated, which adds to the carbon emissions, and the remaining end up either in landfills or water bodies along with the active finishing chemicals. Moreover, the clothes finished with such anti-microbial and hygiene finishes are laundered in households, hospitals, etc. The wastewater from laundering runs down the drain. Additionally, the wastewater at the end of the wet processing stage i.e. the finishing liquor, emerges out of textile mills and process houses and finds pathways in the ecological systems. The fate of antimicrobials at the end of the garment life cycle and the chemical changes it undergo once it is disposed of in the environment is rarely known. Some of the findings from ecological studies are very alarming.
Inorganic hygiene finishes
Inorganic Antimicrobials refer to metal and metal derivatives used to finish textile substrates. The development of metal salt finishes for textiles is evident since world war when a mixture of copper in chlorinated waxes, and antimony salt was used in military fabrics like tents and truck covers to prevent them from microbial colonization [2]. During those days the side effects of antimicrobials were not known. Since the beginning of the 1960s chemists and ecologists started making focused efforts to produce safer antibiotics.
Recent research has focused on using metals as NPs (Nano-Particles) rather than their conventional use as metal and metal salts as antigerm moieties. Many metal derivatives especially Copper, Zinc, Cobalt have been investigated for antimicrobial finishing. Silver has been the most popular choice among metals for the purpose of hygiene finishing of textiles.
Most of the commercial hygiene finishing agents for fabrics are based on silver chemistry. Ongoing research on the development of antimicrobials flourished during the recent pandemic. Silver ion (Ag+) has been proven to be a broad-spectrum anti-germ agent showing antifungal, antimicrobial, and antibacterial properties on treated apparel, home furnishings, and technical textiles (Figure 1).
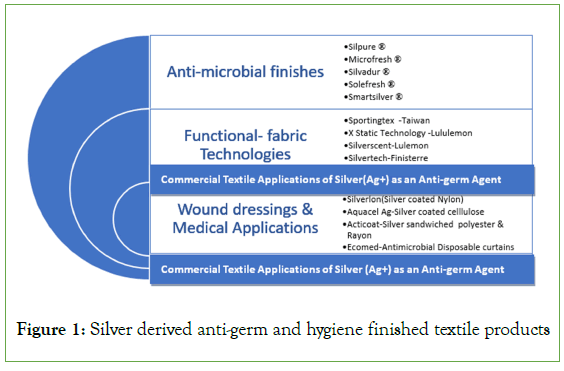
Figure 1: Silver derived anti-germ and hygiene finished textile products
The many commercial textile applications pertaining to the use of Silver as an anti-microbial agent are represented in Figure 1. The increasing use of Silver in textile products has led to major Environmental and health concerns which are lesser known. In a medical review titled, ‘’Silver in Wound Care-Friend or Foe?” researchers have highlighted the prolonged use of Silver as an antimicrobial agent in wound dressings 1 to be toxic to keratinocytes and fibroblasts [3,4].
Moreover research studies have demonstrated that clothes finished with Silver antimicrobials tend to release silver in ionic form when they come in contact with fluids like sweat, saliva and urine [5]. The chances of silver ingestion through dermal contact is more in case of compromised skin like burns, scalds and wounds than through healthy intact skin [6]. The translocation of Ag in the other parts of body is possible via the wounds exposed to such anti-microbial dressings. Cases of Argyria–a blue/grey discoloration of skin caused by deposition of silver salts and prolonged ingestion of silver in the human body, have also been reported in the past [7]. The potential risks of in vivo dermatitis, neurotoxicity, genotoxicity resulting from dermal exposure to silver ions, metallic silver and Ag has also been widely investigated recently.
Organic anti-microbial finishes
Triclosan: Another popular anti-microbial used in textile finishing is triclosan. Recent awareness about its toxicity has led to limited use of Triclosan in antimicrobial finishing. But still there is research happening using triclosan as an antimicrobial agent. For instance cotton, polyester fabrics were modified using triclosan to get biocidal finish [8]. Literature shows many commercial antigerm agents derived from triclosan chemistry. Owing to the lipophilicity of triclosan it readily accumulates in the fats of aquatic creatures [9]. High ppm of triclosan is detected in fat cells of fishes and earthworms [10]. Endocrine-disrupting effects were found in Gambusia Affinis, a species of male mosquito fish [11]. Apart from textiles triclosan was being used in other FMCG products like toothpaste, cosmetics, soaps, sanitizers and hygiene products as well. Wide use of this antimicrobial has far-reached effects, it was detected in wild dolphins [12]. Because of the far-reaching toxic effects of TCS, FDA imposed a ban on using triclosan in personal care products. However, other products like clothes, soft toys, and bedsheets containing TCS are not governed by FDA. TCS was also found in samples of breast milk. Due to its alarming presence in biological systems, it has been marked as ‘Emerging Pollutant’ [13]. A recent study claimed that triclosan transforms into Methyltriclosan which is more toxic, persistent, and bio-accumulative (Figure 2) [14].
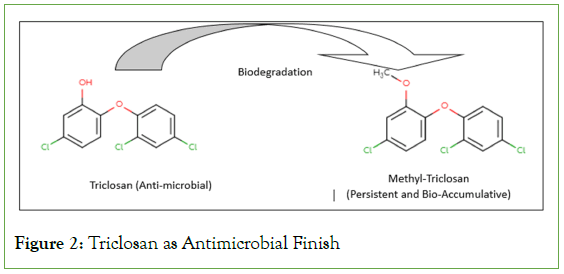
Figure 2: Triclosan as Antimicrobial Finish
Another mesocosm analysis on crabs proved that micro-plastic polyethylene infected with Triclosan is harmful to aquatic beings as it acts like a carrier for micro-plastic in water bodies [15].
Quaternary Ammonium Compounds (QAC): QAC’s are another class of anti-microbials used for finishing mainly protein fibres. They show antibacterial activity against both gram-positive and gram-negative bacteria and they are also virucidal in nature [16]. The positively charged nitrogen cation with long alkyl chain {R4N+X−} clings to an anionic fiber surface via weak ionic interaction. These weak bonding between the finishing agent and the fiber leads to easy leaching of finishing agent from fabrics [17]. Such finishes tend to come out easily post laundering. QACs are also lethal to aquatic life. Due to their overall cationic charge, they absorb readily onto soil, sediments and negatively charged cell wall of algae. Several animal models have confirmed toxicity from QAC’s [18].
N-Halamines: These are one of a kind regenerative anti-microbials which can be used for finishing fabrics. In the presence of water, the Nitrogen–Halogen covalent bond gets reduced. This reaction is reversible. On washing with a mild chlorine bleach their anti-bacterial activity is restored (Figure 3) [19].
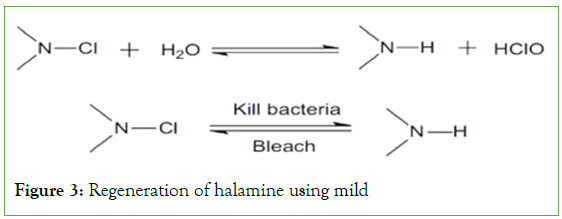
Figure 3: Regeneration of halamine using mild
Initially n-Halamines were applied using cross linkers like DMDHU to produce wrinkle free and antibacterial functional fabrics [20]. However, the cross-linkers were a concern as they released free formaldehyde (carcinogen) when in use. Apparently, formaldehyde free, reactive n-Halamines, cyclic and halamine silanes/siloxanes copolymers and graft polymers have been coated on fabrics to get antimicrobial properties. However, the main disadvantage of n-halamine chemistries is the requirement of chlorination (bleach) to regenerate the anti-microbial activity producing oxidative halogen. This regeneration process which is done by exposure to free halogens often results in change in shade/discoloration of fabrics and an undesirable odor [20].
Polyhexamethylene Biguanide (PHMB): It is often cited as an anti-microbial with relatively less toxicity. But large amounts of active compound are required for finishing which makes it process in-efficient with respect to cost [21].
Anti-microbial finishes derived from natural sources: Chitosan derived from chitin, a naturally occurring polymer has been studied widely to functionalize textiles with antibacterial properties. The application of chitosan as a hygiene finish results in undesirable handle of fabric which compromises the comfort of wearer [22]. Many other natural extracts of neem, tea tree oil, clove, aloevera have also been investigated. Enhancing the durability of such natural finishes and poor fastness to laundering are major challenges in their application scalability [23].
Discussion
Problems caused by antigerm and hygiene finishing of textiles
A three-step effluent treatment is generally employed for textile effluent discharged from wet processing mills. Initially the effluent is screened for particulate matter like fibre lint, floating matter like grease, sizing agents like CMC, Starch, PVA used in pretreatment of textiles. The suspended solids and settled matter are removed by employing gravimetric sedimentation, flocculation and coagulators. After the primary treatment the effluent is subjected to secondary effluent treatment system which uses the metabolic processes of biological organisms to remove remaining waste. Secondary waste treatment using biological agents can be incorporated via three mechanisms-aerobic, anaerobic and anoxic. Bacteria and microbes digest the remaining waste either in presence of oxygen (aerobic) or in absence of oxygen (anaerobic). The waste organic matter gets converted to CO2, water and other microbes as end products (Figure 4) [24].
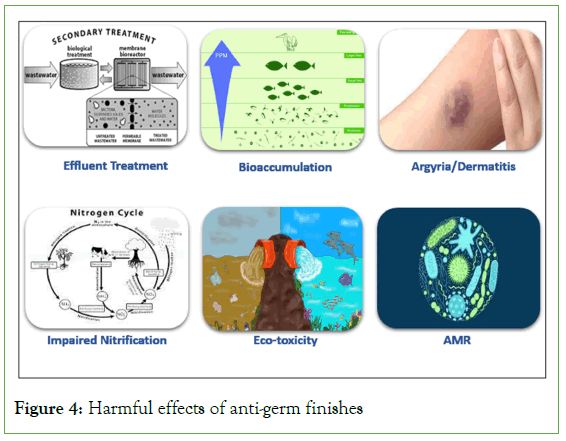
Figure 4: Harmful effects of anti-germ finishes
Anti-microbial finishing chemicals which are present in the finishing liquor become a part of the effluent stream reaching the ETP. These antimicrobial finishing chemicals can have an anti-effect on the micro-organisms present in the secondary effluent treatment process. The presence of antimicrobial finishes in the effluent can disrupt the metabolic functions of microbes which serve the purpose of secondary waste treatment. Special separators and sophisticated technologies like ozonation, advanced oxidation need to be employed for separating the antimicrobial moieties from the effluent prior to its flow into secondary biological treatment plant.
Effect on nitrogen cycle
The multiple applications of Silver Nano Particles in hygiene finishes and Anti-microbial agents can be understood by representation in Figure 5. Till date there have been several studies reported with regard to the toxicity of Silver Nano particles and their impact on ecological systems. The ever-increasing presence of silver nano particles in PPC (Personal Protective Care) products has led to their distribution in various environmental strata’s. Silver nano-particles have been the first choice for anti-microbial finishing of fabrics in wake of COVID-19 pandemic. Approximately 10 nm in size Ag-Nps have shown antiviral properties against SARS COVID Virus.
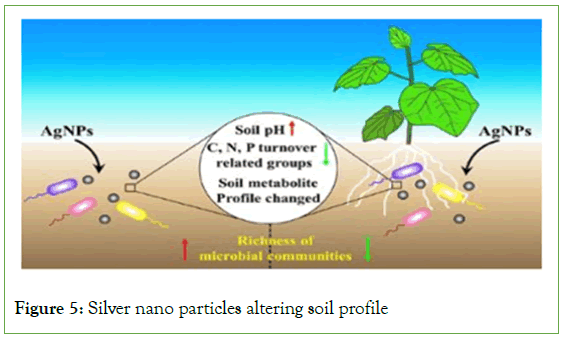
Figure 5: Silver nano particles altering soil profile
The nitrogen cycle is an important geochemical process that replenishes nitrogen in soil. Studies have confirmed the presence of Ag-NPs in soil. They are known to have detrimental effects on soil nutrition. Ag-Nps impairs the nitrification process in the nitrogen cycle by affecting the biological communities involved in N, P, C metabolisms (Figure 5).
Antimicrobial Resistance (AMR)
When micro-organisms get immune to antimicrobial action and no longer respond to antimicrobial use. Drug resistant genes are developed on prolonged exposure to anti-microbials. It is a condition where bacteria, fungi, parasites and viruses become adaptable to the use anti-microbials. Such conditions make it more difficult to cure infections and result in transmission of life-threatening diseases faster. AMR is a major challenge which is not very often addressed by the textile industry. The spread of antimicrobial resistant germ pools is a result of excessive use of antimicrobials.
The overuse of anti-biotics in pharmaceuticals, personal care and hygiene products like soaps, sanitizers, body washes, disinfectants, cleansers is often cited as the main cause of anti-microbial resistance. However, the use of antimicrobial, antiviral, anti-fungal hygiene finishes used in textile goods should also be taken into consideration. According to World Health Organization, AMR is among the top ten risks posed to public health and well-being [25].
Summary
Statistical analysis has shown that communicable diseases pose a threat to 1.2 billion people in India and every third death in the world is because of an infectious disease [26]. Recent studies by researchers across the globe surveying healthcare and hospitality workers have proved that textiles impregnated with antimicrobials provide bio-safety and act like barriers against HAIs [27]. Textiles having antimicrobial functionality are not only limited to clothes but also find applications in other technical textile segments.
The eco-toxicity and safety concerns of antimicrobials have been a debatable topic across many forums. Finding viable alternatives to existing textile antimicrobial finishes is an emerging field of research. Recent medicinal literature has shown the use of medical grade honey having low pH and sustainable broad spectrum antibacterial activity to be a viable substitute to silver nanoparticles in wound dressings [28]. Especially to manage wounds from burns and scalds honey has shown faster epithelialization without causing cytotoxicity. Sustainable use of OCT (Octenidine Dihydrochloride) has been reported as a substitute to silver nanoparticles in wound care dressings [29].
Most of the anti-microbial agents used for finishing textiles and the antimicrobial/anti-biotic ingredients used in pharmaceuticals and PPC products are based on similar chemistries. The end-of-life cycle and the fate of antimicrobials once they find pathways in external environment needs more detailed data based evaluation. The ultimate consequences like AMR, contaminated soil, eco-toxicity can be controlled by responsible use.
Conclusion
Based on the information gathered from various sources pertaining to the available chemical components and the end requirements, it is apparent that further research is necessary on test methods to determine the exact Minimum Inhibitory Concentration (MIC) values with regard to the application of antimicrobials on textiles, striking a balance between sufficing the need of antimicrobial activity and avoiding the risk of AMR. Consumers also need to be more aware about the hygiene wear and fabrics finished with anti-microbials. Commercially ‘odor free’, ‘perma stink resistant’ apparel, athleisure wear, antibacterial intimate wear is being sold as commoditized products. The consumers should be aware of such product profiles. Such garments finished with antimicrobials are intended for a longer product life requiring lesser number of washes. The anti-germ and hygiene finished textiles require less wash cycles as compared to the conventional clothes. However, the lack of awareness among consumers results in fewer washing similar to conventional fabrics.
The garment manufacturers should provide accurate information to consumers on product care and wash cycles required for such fabrics finished with anti-microbials. This can effectively increase the product’s life as less washes will result in less pilling, lesser lint, lesser fading. This will in turn benefit energy and water savings, reducing footprints and the effluent load which goes down the drain. While aiming for biosafety of human health it’s crucial to be concerned about ecological safety as well.
Acknowledgement
The authors would like to acknowledge the support and cooperation received from the Institute of Chemical Technology Mumbai and the Department of Science and Technology program on Innovation in Science in Pursuit for Inspired Research.
Conflict of Interest
All authors agree for the publication. For all authors, there is no competing interests to declare.
References
- Akca C. The waste problem of antimicrobial finishing. InWaste in Textile and Leather Sectors. 2020.
- Gulati R, Sharma S, Sharma RK. Antimicrobial textile: Recent developments and functional perspective. Polym Bull. 2022;79(8):5747-5771.
[Crossref] [Google Scholar] [PubMed]
- Khansa I, Schoenbrunner AR, Kraft CT, Janis JE. Silver in wound care—friend or foe?: A comprehensive review. Plast Reconstr Surg - Glob Open. 2019;7(8).
[Crossref] [Google Scholar] [PubMed]
- Beam JW. Topical silver for infected wounds. J Athl Train. 2009;44(5):531-533.
[Crossref] [Google Scholar] [PubMed]
- Stefaniak AB, Duling MG, Lawrence RB, Thomas TA, LeBouf RF, Wade EE, et al. Dermal exposure potential from textiles that contain silver nanoparticles. Int J Occup Environ Health. 2014;20(3):220-234.
[Crossref] [Google Scholar] [PubMed]
- Trop M, Novak M, Rodl S, Hellbom B, Kroell W, Goessler W. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma Acute Care Surg. 2006;60(3):648-652.
[Crossref] [Google Scholar] [PubMed]
- Vlachou E, Chipp E, Shale E, Wilson YT, Papini R, Moiemen NS. The safety of nanocrystalline silver dressings on burns: a study of systemic silver absorption. Burns. 2007;33(8):979-985.
[Crossref] [Google Scholar] [PubMed]
- Orhan M. Triclosan applications for biocidal functionalization of polyester and cotton surfaces. J Eng Fibers Fabr. 2020;15:1558925020940104.
- Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health B Crit. Rev. 2017;20(8):447-469.
[Crossref] [Google Scholar] [PubMed]
- Gillis JD, Price GW, Prasher S. Lethal and sub-lethal effects of triclosan toxicity to the earthworm Eisenia fetida assessed through GC–MS metabolomics. J Hazard Mater. 2017;323:203-211.
[Crossref] [Google Scholar] [PubMed]
- Raut SA, Angus RA. Triclosan has endocrine‐disrupting effects in male western mosquitofish, Gambusia affinis. Environ Toxicol Chem. 2010;29(6):1287-1291.
[Crossref] [Google Scholar] [PubMed]
- Bever CS, Rand AA, Nording M, Taft D, Kalanetra KM, Mills DA, et al. Effects of triclosan in breast milk on the infant fecal microbiome. Chemosphere. 2018;203:467-473.
[Crossref] [Google Scholar] [PubMed]
- Yin Y, Wu H, Jiang Z, Jiang J, Lu Z. Degradation of triclosan in the water environment by microorganisms: A review. Microorganisms. 2022;10(9):1713.
[Crossref] [Google Scholar] [PubMed]
- Contardo-Jara V, Meinecke S, Feibicke M, Berghahn R, Schmidt R, Mohr S. Fate, bioaccumulation and toxic effects of triclosan on a freshwater community–A mesocosm study. Environmental Advances. 2021;5:100100.
- Nobre CR, Moreno BB, Alves AV, de Lima Rosa J, Fontes MK, de Campos BG, et al. Combined effects of polyethylene spiked with the antimicrobial triclosan on the swamp ghost crab (Ucides cordatus; Linnaeus, 1763). Chemosphere. 2022;304:135169.
[Crossref] [Google Scholar] [PubMed]
- Morrison KR, Allen RA, Minbiole KP, Wuest WM. More QACs, more questions: Recent advances in structure activity relationships and hurdles in understanding resistance mechanisms. Tetrahedron Lett. 2019;60(37):150935.
[Crossref] [Google Scholar] [PubMed]
- Mohapatra S, Yutao L, Goh SG, Ng C, Luhua Y, Tran NH, et al. Quaternary ammonium compounds of emerging concern: Classification, occurrence, fate, toxicity and antimicrobial resistance. J Hazard Mater. 2023;445:130393.
[Crossref] [Google Scholar] [PubMed]
- Hora PI, Pati SG, McNamara PJ, Arnold WA. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: Consideration of environmental implications. Environ Sci Technol Lett. 2020;7(9):622-631.
[Crossref] [Google Scholar] [PubMed]
- Ren X, Jiang Z, Liu Y, Li L, Fan X. 8-N-halamines as antimicrobial textile finishes. In Antimicrobial Textiles. Woodhead Publishing. 2016:125-140.
- Li R, Sun M, Jiang Z, Ren X, Huang TS. N-halamine-bonded cotton fabric with antimicrobial and easy-care properties. Fibers Polym. 2014;15:234-240.
- The waste problem of antimicrobial finishing
- Dhiman G, Chakraborty JN. Antimicrobial performance of cotton finished with triclosan, silver and chitosan. Fashion and Textiles. 2015;2(1):13.
- Naebe M, Haque AN, Haji A. Plasma-assisted antimicrobial finishing of textiles: A review. Engineering. 2022;12:145-163.
- Kumar A, Nath K, Parekh Y, Enayathullah MG, Bokara KK, Sinhamahapatra A. Antimicrobial silver nanoparticle-photodeposited fabrics for SARS-CoV-2 destruction. Colloids Interface Sci Commun. 2021;45:100542.
[Crossref] [Google Scholar] [PubMed]
- Crossref
- Ram B, Thakur R. Epidemiology and economic burden of continuing challenge of infectious diseases in India: analysis of socio-demographic differentials. Front Public Health. 2022;10:901276.
[Crossref] [Google Scholar] [PubMed]
- Schneider G, Vieira LG, Carvalho HE, Sousa ÁF, Watanabe E, Andrade DD, et al. Textiles impregnated with antimicrobial substances in healthcare services: Systematic review. Front Public Health. 2023;11:1130829.
[Crossref] [Google Scholar] [PubMed]
- Tashkandi H. Honey in wound healing: An updated review. Open Life Sci. 2021;16(1):1091-1100.
[Crossref] [Google Scholar] [PubMed]
- Ciecholewska-Juśko D, Junka A, Fijałkowski K. The cross-linked bacterial cellulose impregnated with octenidine dihydrochloride-based antiseptic as an antibacterial dressing material for highly-exuding, infected wounds. Microbiol Res. 2022;263:127125.
[Crossref] [Google Scholar] [PubMed]
Citation: Tari S, Athalye A (2023) Anti-germ and Hygiene Finishing of Textiles for Human Health and Safety. Modern Chem App. 11:425.
Copyright: © 2023 Tari S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


