Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2021) Volume 12, Issue 7
Antibacterial Effect of Pongamia pinnata Leaf Extract against Some Human Pathogenic Bacteria
Aviral Asaiya*, Diwyansh Raj and Chokhelal PrajapatiReceived: 15-Apr-2021 Published: 29-Jul-2021, DOI: 10.35248/2157-7471.21.12.565
Abstract
Medicinal plants play an important role in the discovery of novel drugs used in modern medicine. The medicinal plant Pongamia pinnata leaf possesses the wide range of medicinal properties which were confirmed through literature reviews. The leaves of the Pongamia pinnata have been used for the medicinal purposes since ancient time research has suggested that Pongamia pinnata leaf extracts have antimicrobial properties. The present study was to determine its leaves have any antibacterial activity leaf aqueous extract were screened for against different species of human pathogens, Chromobacterim violaceum, Citrobacter frendii, Staphylococcus aureus and Micrococcus luteus. The activity of leaf aqueous extracts was measured by agar well diffusion method. Nutrient Agar Media (NAM) was prepared for growth of bacteria strains; leaf aqueous extract was obtained by mixing the pulverized leaf materials with ethanol, filtered with Whatman No. 1 filter paper and concentrated to dryness. The collected extracts were tested for antibacterial activities, after that plates placed in incubator for incubation at 37 ± 2°C for 24 hours. Measure the zones of inhibition obtained. The obtained data were statistically analyzed by using standard deviation and standard error. The highest zone of inhibition of P. pinnata ethanolic leaf aqeous extract was measured against gram-positive bacteria Micrococcus luteus (38 mm) and smallest was measured against Citrobacter freundii (17.6 mm). In this present study we have evaluated the efficacy of P. pinnata leaf extract against four different human pathogenic bacteria.
Keywords
Antimicrobial activity; Pongamia pinnata; Human pathogen; Medicinal plant; Agar well diffusion
Introduction
unmet therapeutic needs [5]. It is hoped that study would leads to the establishment of some compound that used to formulate new and more potent antimicrobial drug or natural origin. Many herbal remedies individually or in combination have been recommended in various medical treatises for the cure of different diseases [6].Different parts of the plant have been used in traditional medicine for bronchitis, whooping cough rheumatic joints and to quench dipsia in diabetes. Leaves are hot digestive, laxative, anthelminitic and cure piles, wounds and other inflammations [7]. A hot infusion of leaves is used as a medicated bath for relieving rheumatic pain and for cleaning ulcer in gonorrhea and scrofulous enlargement different extract of leaves, roots and seeds are used to treat infection diseases such as leucoderma, leprosy, lumbago, muscular and articular rheumatism [8]. Fruits and sprouts of P. pinnata were used in folk remedies for abdominal tumours in India and seed extract has hypontensive effects and produce uterine contractions. Flowers are prescribed for glycosuria and diabetes [9]. The bark is used internally for bleeding piles, beriberi and as an antimicrobial. Karanja seed is used as a medicinal plant, particularly with the Ayurvedic and siddha medicine system of India [10]. Leaves are active against micrococcus, their juice are use for cold, cough, diarrohea, dyspepsia, gonorrhoea and leprosy and seed oil is used in itches, abscess, and other skin diseases [11]. Roots are used for cleaning gums, teeth and ulcers. Powdered seeds are valued as febrifuge, tonic and in bronchitis [12] and examined in vitro for antiplasmodial properties against Plasmodium falciparum. Ethanol extract of P. pinnata shows significant anti plasmodial activity [13] and leaf extract of P. pinnata shows circulatory lipid per oxidation and antioxidant activity [14].
Choromobacterium violaceum is a gram-negative, facultative anaerobic that can grow without need of oxygen, non-sporing coccobacillus [15]. It is motile with the help of single flagellum. It is part of the normal flora water and soil of tropical and sub-tropical regions of the word. C. violaceum rarely infected humans, but when it does it cause skin lesions, sepsis and liver abscesses that be fatal [16]. To date cause have been reported from Argentina, Australia, Brazil, Canada, Cube, India, Japan, Nigeria, Singapore, Shri Lanka, Taiwan, United states and Vietnam [17]. The disease usually started as a limited infection of the skin of the point of entry of the bacteria which progress to necrotizing metastatic lesions then multiple abscesses of the liver, lungs, spleen, skin, lymph nodes or brain, leading to severe septicemia, culminating in multi organ failure which may be fatal [18].
Citobacterfreundiiis a gram-negative, facultative anaerobes, belongs to Entrebacteriacea family. The bacteria have a long rod shape. C. freundii have several flagella used for the locomotion, but some do not and non-motile. C. freundii is found in soil but can also be found in water, sewage, food and in the intestinal tracts of animal and human [19], C. freundii caused urinary tract infection, healthcare associated infection, especially pediatric and immunocompromised patients, muco hemorrhagic diarrhea in animals being bacteremic and septicemia many organs and tissues are affected besides the gut [20].
Staphylococcus aureus is a gram-positive, round shaped, non-motile and does not form spores, facultative anaerobes, belongs to the family Staphylococcaceae. It is a member of the micro-biota of the body [21]. S. aureus usually acts as a commensally bacterium asymptomatically colonizing about 30% of the human population, it can sometime causes infection like respiratory infection such as sinusitis, food poisoning [22]. Bacteremia and infective endocarditic additionally it can various skin and soft tissue infection including abscesses.
Micrococcus luteus is a gram-positive to gram-variable non-motile, coccus tetrad-arranging, pigmented, saprotrophic [23]. It is catalase positive and obligate aerobe [24]. That belongs to Micrococcales family. M. luteus found in soil, dust, water and air and as part of the normal micro-biota of the mammalian skin. M. luteus causes diseases are brain abscesses, Central nervous system infections [25].
Materials and Methods
Sample collection and sterilization
Pongamiapinnata healthy leaves were collected from the field of Tropical Forest Research Institute, Jabalpur (M.P.) Surface sterilization of leaves with HgCl2 (0.1%) for a duration of 6 minutes significantly reduced microbial contamination (7.78%) but also reduced the survival percentage (51.77%) and caused drying of explants within 10 days of inoculation which may be due to phytotoxicity caused by long exposure of leaves with HgCl2. All the treatment was statistically significantly different to each other [26]. leaves washed with household detergent to remove the impurities and then washed thoroughly under tap water and then sterilization was done with 70% alcohol for 2 minutes followed by 1% HgCl2 for 2~3 minutes [27].
Leaf extract preparation
The extractions were done by following the methods mentioned elsewhere with slight modification [28]. For ethanol extraction, sample was mixed with ethanol at a ratio of 2:10 into sterile flask and was placed in orbital shaker water bath at 130r/min at 37°C overnight. The liquid samples were then filtered with Whatman No.1 filter paper. The extracted samples were stored in universal bottles and refrigerated at 4°C prior to use.
Culture media
The media use for the study was Nutrient Agar Medium (NAM) used for the bacteria growth.
Agar well diffusion assay
The well diffusion assay was used for testing antimicrobial activity. Each sterilized Petri plate was pre-seeded with 15 ml of respective growth agar medium and 80μl of bacterial culture and lawn was prepared by spread plate method to dry for 30 minutes, and then make wells with the help of sterile cork borer, prepared wells numbers was (A) Leaf aqueous extract (50 µl), (B) Blank, (C) Negative control (ethanol 70%) (50 µl) and last one was (D) Positive control (Antibiotic) so as to allow the diffusion of the substances and then incubated at 37±2ºC for 18-24 hours in case of bacteria [29]. Measure the zones of inhibition obtained; the obtained data were statistically analysed by using standard deviation and standard error.
Statistical analysis
This Experiment was performed in triplicate in an independent manner. The data were expressed as mean and standard error of three replicates and values were analysed statistically (Figure 1).
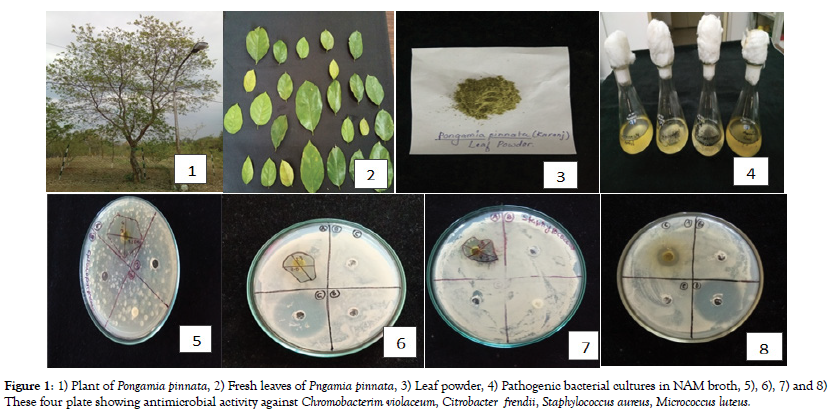
Figure 1. 1) Plant of Pongamia pinnata, 2) Fresh leaves of Pngamia pinnata, 3) Leaf powder, 4) Pathogenic bacterial cultures in NAM broth, 5), 6), 7) and 8) These four plate showing antimicrobial activity against Chromobacterim violaceum, Citrobacter frendii, Staphylococcus aureus, Micrococcus luteus.
Results and Discussion
Antibacterial activity of leaf extract of Pongamia pinnata was evaluated against following pathogenic bacterial strains Micrococcus luteus, Citrobacter freundii, Staphylococcus aureus, and Chromobacterium violaceum both gram positive and gram negative bacteria has produced after 24 hours of incubation. It has found that leaf extract has showed minimum inhibitory concentration (MIC) against Micrococcus luteus was 38 mm, Citrobactor frendii 17.6 mm, Staphylococcus aureus 37.8 mm and Chromobacterium violaceum 33.6 mm (Table 1).
| Human pathogenic bacteria | Zone of inhibition* (in mm.) | ||
|---|---|---|---|
| Negative control (Ethanol) |
Leaf extract | Positive control (Streptomycin) |
|
| Micrococcus luteus | - | 38 | 85 |
| Citrobacter freundii | - | 17.6 | 79 |
| Staphylococcus aureus | - | 37.8 | 85 |
| Chromobacterium violaceum | - | 33.6 | 90 |
Table 1: Zone of inhibition of Pongamia pinnata leaves extract in millimeters (mm).
The traditional use of plants provides the basis for indicating the type of plant extracts useful for controlling various pathogenic microorganisms. Historically, many plant extracts have antimicrobial properties [12]. Also the renewal of interest in the food industry and increasing consumer demand for effective, safe, natural products means the qualitative data on plant extracts are well documented [5]. Therefore, plant extracts are known to be the promising antimicrobial agents to inhibit the bacterial pathogens. The leaves and stem of Pongamia pinnata consist of several flavones, chaconne derivatives [30]. Reported the antimicrobial activity of flavonoids from leaf extract of Pongamia pinnata but it did not show activity against S. epidermis, M. smegmatis, S. typhimurium, and C. candida. That study to the lead to the establishment of some compound that used to formulated new and more potent antimicrobial drug against antibiotic resistant human and plant pathogens [31].
Conclusion
The leaves of Pongamia pinnata are Antioxidant and Anti- hypermmonemic and have also Anti-diarrheal activity. The result of the antibacterial screening showed that leaf extract of ethanol have potential antibacterial effects against some pathogens: M. luteus, C. frendii, S. aureus and C. violaceum. Pongamia pinnata mediated organic extracts could be a source of natural antimicrobial agents for use in food or pharmaceutical industries to control food-borne pathogenic bacteria. Further study is in preparation to evaluate the bioactive compounds present in various organic extracts Pongamia pinnata are to be used for drug or food preservation, issues of safety and toxicity will always need to be addressed.
Acknowledgements
Authors are thankful to the Director, TFRI Jabalpur for encouragement and support to carry out this work.
REFERENCES
- Arote SR, Yeole PG. Pongamia pinnata L: A Comprehensive Review. Int J Pharm Tech Res. 2010;2(4):2283-2290.
 Dhanavade MJ, Jalkute CB, Ghosh JS, Sonawane KD. Study antimicrobial activity of Lemon (Citrus lemoni) peel extract. Br J Pharmacol Toxicol. 2011;2(3):119-122.
Dhanavade MJ, Jalkute CB, Ghosh JS, Sonawane KD. Study antimicrobial activity of Lemon (Citrus lemoni) peel extract. Br J Pharmacol Toxicol. 2011;2(3):119-122.- Bala M, Nag TN, Kumar S, Vyas M, Kumar A, Bhogal NS. Proximate composition and fatty acid profile of Pongamia pinnata, a potential biodiesel crop. J Am. Oil Chem' Soc. 2011;88(4):559-62.
- Jyotsna J, Bechan S. A Comparative Study of Antimicrobial and Pharmacological Properties of Argemone mexicana, Solanum xanthocarpum and Thevetia peruviana. Acta Scientific Microbiology. 2020;3(3): 01-05.
- Yadav RD, Jain SK, Alok S, Prajapati SK, Verma A. Ponagamia pinnata: An overview. Int J Pharm Sci Res. 2011;2(3):494-500.
- Chopade VV, Tankar AN, Pande VV, Tekade AR, Gowekar NM, Bhandari SR, et al. Pongamia pinnata: Phytochemical constituents, Traditional uses and Phytochemical properties. Int J Green Pharm. 2007;72-75.
- Nitika S, Jyotsna J, Priyanka T, Sharma B. Phytochemicals from Citrus Limon Juice as Potential Antibacterial Agents. The Open Bioactive Compounds Journal. 2020;8(1):1-6.
- Manigauha A, Patel S, Monga J, Ali H. Evaluation of anticonvulsant activity of Pongamia pinnata Linn in experimental animals. Int J Pharmtech Res. 2009;1(4):1119-1121.
- Meher LC, Naik SK, Das LM. Methanolysis of Pongamia pinnata (Karanj) oil production of biodiesel. J Sci Ind Res India. 2004;63:913- 918.
- Chitopo W, Manyogi R, Muchachaa R. Evaluation of the antimicrobial activity of the Erythrina abyssinica leaf extract. J Microb Biochem Technol. 2019;11(2):413.
- Wagh P, Rai M, Deshmukh SK, Durate MC. Bio-activity of oil Trigonella Foenum-graecum and Pongamia pinnata. Afr J Biotechnol. 2007;6(13):1592-1596.
- Badole SL, Zanwar AA, Khopade AN, Bodhankar SL. In vitro antioxidant and antimicrobial activity cycloart-23-ene-3 B, 25-Diol (B2) isolated from Pongamia pinnata (L. Pierre). Asian Pac J Trop Med. 2011;4(11):910-916.
- Sangwan S, Rao DV, Sharma RA. Biochemical estimation of primary metabolites from pongamia pinnata (L.): An important Biodiesel Plant. Int J Pharmaceut Rev Res. 2010;5(1).
- Scott PT, Pregelj L, Chen N, Hadler JS, Djordjevic MA, Gresshoff PM. Pongamia pinnata- An Untapped Resource for the Biofuels Industry of the Future. Bioenerg Res. 2008;1:2-11.
- D'almeida RE, Molina RD, Viola CM, Luciardi MC, Peñalver CN, Bardón A, et al. Comparison of seven structurally related coumarins on the inhibition of quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorg Chem. 2017;1(73):37-42.
- Ravichandran V, Zhong L, Wang H, Yu G, Zhang Y, Li A. Virtual screening and biomolecular interactions of CviR-based quorum sensing inhibitors against Chromobacterium violaceum. Front Cell Infect Microbiol. 2018;48:292.
- Chang A, Sun S, Li L, Dai X, Li H, He Q, et al. Tyrosol from marine Fungi, a novel Quorum sensing inhibitor against Chromobacterium violaceum and Pseudomonas aeruginosa. Bioorg Chem. 2019;91:103140.
- Devescovi G, Kojic M, Covaceuszach S, Cámara M, Williams P, Bertani I, et al. Regulation of violacein biosynthesis in Chromobacterium violaceum. Frontiers in microbiology. 2017;7(8):349.
- Anderson MT, Mitchell LA, Zhao L, Mobley HL. Citrobacter freundii fitness during bloodstream infection. Scientific reports. 2018;8(1):1-4.
- Liu LH, Wang NY, Wu AY, Lin CC, Lee CM, Liu CP. Citrobacter freundii bacteremia: Risk factors of mortality and prevalence of resistance genes. J Microbiol Immunol. 2018;51(4):565-572.
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler Jr VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603-661.
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378).
- Barcenal AR, Traifalgar RF, Corre VL. Anti-Vibrio harveyi property of Micrococcus luteus isolated from rearing water under biofloc technology culture system. Curr Res Bacteriol. 2015;8(2):26-33.
- Nwachukwu U, George-Okafor U, Ozoani U, Ojiagu N. Assessment of probiotic potentials of Lactobacillus plantarum CS and Micrococcus luteus CS from fermented milled corn-soybean waste-meal. Scientific African. 2019;6:e00183.
- Erbasan F. Brain abscess caused by Micrococcus luteus in a patient with systemic lupus erythematosus: case-based review. Rheumatol Int. 2018;38(12):2323-2328.
- Verma S, Yadav K, Singh N. Optimization of the protocols for surface sterilization, regeneration and acclimatization of Stevia rebaudiana Bertoni. J Agric Environ Sci. 2011;11(2):221-227.
- Ilahi I, Jabeen M, Sadaf SN. Rapid clonal propagation of chrysanthemum through embroyogenic callus formation. Pak J Bot. 2004;39:1945-1952.
- Archana S, Abraham J. Comparative analysis of antimicrobial activity of leaf extracts from fresh green tea, commercial green tea and black tea on pathogens. J Appl Pharm Sci. 2011;(8):149-152.
- Mamta G, Diwyansh R, Isha D. Antibacterial and Antioxidant Activity of Biomolecules Extracted from Paecilomyces sinensis â?? An Endophyte of Oroxylum indicum (L.) Vent. J Mycol Pl Pathol. 2020;50(1):57-66.
- Sharma A, Tyagi S, Nag R, Chaturvedi A, Nag TN. Antimicrobial activity cellular toxicity of flavonoid extracts from Pongamia pinnata and Vitex negundo. Rom Biotechnol Lett. 2011;16(4):6396-6400.
- Bajpai VK, Rahman A, Shukla S, Rahman MM, Mehta M. Antibacterial activity of leaf extract of Pongamia pinnata from India. Pharm Biol. 2009;47(12):1162-1167.
Citation: Asaiya A, Raj D, Prajapati C (2021) Antibacterial Effect of Pongamia pinnata Leaf Extract Against Some Human Pathogenic Bacteria. J Plant Pathol Microbiol. 12:565.
Copyright: © 2021 Asaiya A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

