Indexed In
- Open J Gate
- Academic Keys
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- Google Scholar
- SHERPA ROMEO
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2021) Volume 9, Issue 1
Analysis of the Architectural Characteristics of the Abdominal Aortic Aneurysm in Dizygotic Twins
Maged Metias1*, Anand Patel2, Vikram Iyer1,2 and Theodore Rapanos1,22Michael G. DeGroote School of Medicine, McMaster University, Hamilton Ontario, Canada
Received: 20-Nov-2020 Published: 08-Jan-2021, DOI: 10.35248/2329-6925.9.37.402
Abstract
We report a case of dizygotic twins who were diagnosed with abdominal aortic aneurysms (AAAs) and analyze the differences in architectural characteristics of the abdominal aorta and visceral vessels. Both twins underwent an endovascular repair of their AAAs at the same age. This paper adds to the growing body of evidence of the genetic nature of aneurysmal degeneration.
Keywords
Abdominal aortic aneurysm; Dizygotic; Architectural characteristics; Dizygotic twins
Introduction
Negating collagen vascular diseases, family history of Abdominal Aortic Aneurysms (AAAs) along with age, smoking, atherosclerosis areeach independent risk factors for the development of AAAs [1]. Some estimate that 15 percent of patients with AAAs have a positive family history of the pathology [2]. The proportion of individuals with a familial AAA is approximately 13 percent, in which 17% of men with a relative with an AAA were also found to have an AAA on ultrasound surveillance [3]. One study found that familial AAAs were most common between brothers [4]. Here we discuss anatomic variations in the architecture of the AAA in dizygotic twin brothers who underwentan endovascular intervention at the same age. Consent was obtained from both patients for publication.
Case 1
A 71-year-old male presented to the vascular surgery service after an incidental finding of an asymptomatic 4.3 cm Abdominal Aortic Aneurysm (AAA) found during ultrasound of his bladder.
His past medical history was significant for hypertension, dyslipidemia, type II diabetes, and obstructive sleep apnea with CPAP non-compliance. He was medically managed for these comorbidities while placed on Aspirin 81 mg, Rosuvastatin 10 mg, telmisartan 20 mg, and metformin 500 mg daily. His blood group was O positive and had a positive family history of an abdominal aortic aneurysm in his father. He was an ex-smoker of 50 pack years who quit 6 months prior to his presentation. His height and weight were 182 cm and 103.6 kg, respectively yielding a BMI of 31.3 kg/ m2. His computed tomography (CT) findings are summarized in Table 1 and Figures 1-3. Of note, his presenting aneurysm measured 4.1 × 4.6 cm. His six-month follow-up measurements discovered a rapidly growing AAA measuring 5.9 × 4.3 × 54 cm with extension of the aneurysm into the common iliac arteries (CIAs) measuring 4.2 cm and 2.0 cm on the right and left CIA, respectively. He also had two accessory renal arteries on the left and another on the right as well as a patent inferior mesenteric artery (IMA). He was consented for an endovascular approach to repair the AAA.
| Case 1 | Case 2 | |
|---|---|---|
| Diameter of supra-renal abdominal aorta | 26.6 mm | 24.4 mm |
| Neck length | 49 mm | 3.7 cm |
| Neck diameter | 20.2 mm | 24.0 mm |
| Aneurysm Diameter | 4.1 × 4.6 cm | 7.8 × 7.5 cm |
| Length or AAA | 69.7 mm | 99.56 |
| Distance from lowest renal to bifurcation | 107.34 mm | 143.27 mm |
| Right CIA maximal diameter | 4.2 cm | 2.2 cm |
| Left CIA maximal diameter | 2.0 cm | 2.0 cm |
| Accessory renal | Left | None |
| Clock position of right renal artery | 10:00 | 9:00 |
| Clock position of Left renal artery | 3:00 | 2:30 |
Table 1: Dimensions and anatomical location of the AAAs and visceral vessels.
Intraoperative, the patient had a main body installed (Cook Medical, ZIMB-30-98) with limb extensions into the left (ZSL-24- 59) and right (ZISL-24-59) CIAs.
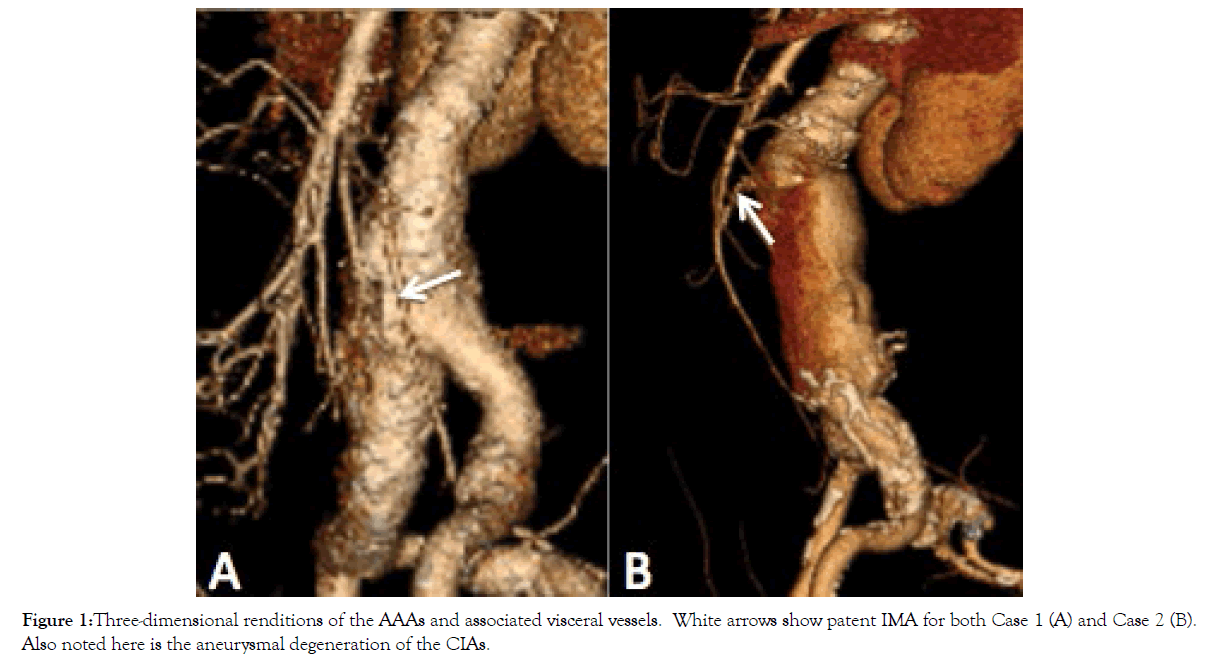
Figure 1: Three-dimensional renditions of the AAAs and associated visceral vessels. White arrows show patent IMA for both Case 1 (A) and Case 2 (B). Also noted here is the aneurysmal degeneration of the CIAs.
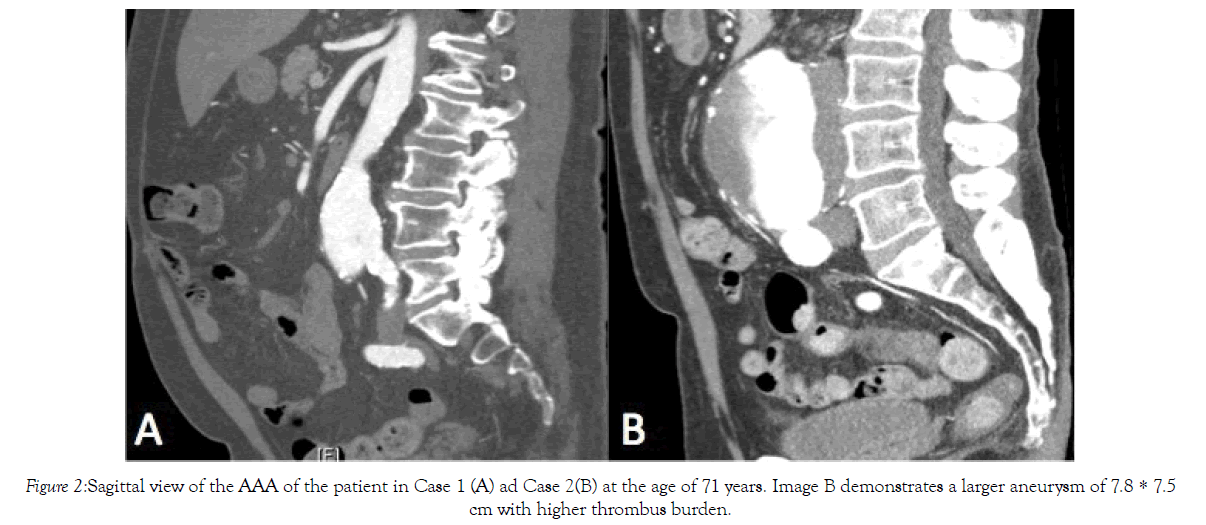
Figure 2: Sagittal view of the AAA of the patient in Case 1 (A) ad Case 2(B) at the age of 71 years. Image B demonstrates a larger aneurysm of 7.8 * 7.5cm with higher thrombus burden.
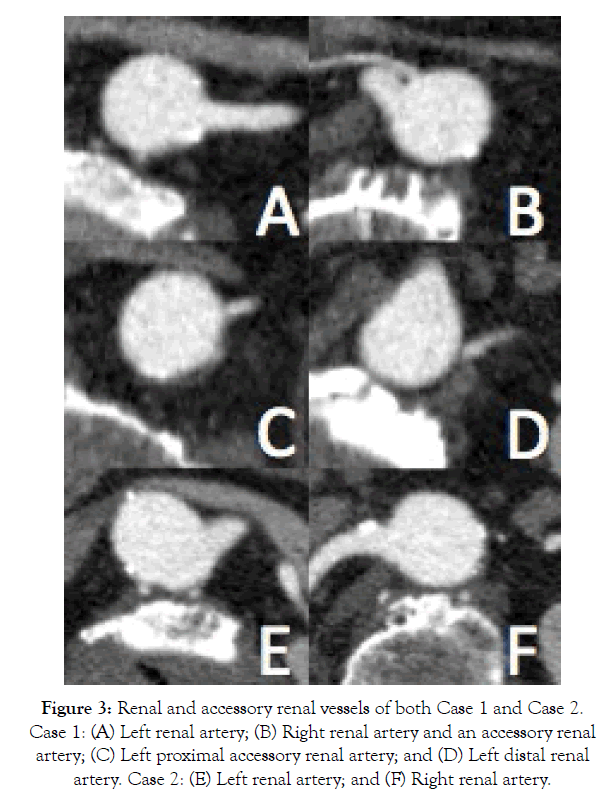
Figure 3: Renal and accessory renal vessels of both Case 1 and Case 2. Case 1: (A) Left renal artery; (B) Right renal artery and an accessory renal artery; (C) Left proximal accessory renal artery; and (D) Left distal renal artery. Case 2: (E) Left renal artery; and (F) Right renal artery.
Case 2
71-year-old twin male underwent an ultrasound in light of his twin brother’s AAA diagnosis. He was discovered to have an 8.2 × 8.3 cm AAA on ultrasound. He was asymptomatic.
His past medical history was significant for hypertension, dyslipidemia, mixed ischemic/nonischemic cardiomyopathy with an ejection fraction of 26%. Myocardial infarction with a stent insertioninto the right coronary artery with subsequent occlusion. Furthermore, the patient was positive for ventricular tachycardia (VT) with a bi-ventricular ICD and subsequent VT ablation, COPD, asthma, depression, gastric-esophageal reflux disease, tonsillectomy, inguinal hernia and resection of a basal cell carcinoma of the skin.
He was medically managed on the following therapies: ramipril 2.5 mg, crestor 20 mg. metoprolol 50 mg BID, amiodarone 200 mg daily, ASA 81 mg, escitalopram 10 mg, fluticasoen 250 mcg, ipratroprium 20 mcg, and omeprazoel 20 mg BID. Like his brother, his blood group was O positive. He was an ex-smoker of 50 pack years who quit 1 year prior to his presentation. His height and weight were 182 cm and 77.2 kg, respectively yielding a BMI of 23.3 kg/m. His CT findings are summarized in Table 1 and Figures 1-3. His imaging revealed an AAA measuring 7.8 × 7.5 cm. His CIAs were dilated bilaterally at 2.2 cm on the right and 2.0 cm on the left. Unlike his brother, he did not have any accessory renal arteries, but similarly had a patent IMA. He requested and was consented for an endovascular repair, which he underwent in that same year.
Intraoperative, the patient had a main body deployed (Cook Medical, Zenith® LP-30-108), along with limb extensions into the left (Alpha 24-93) and right (Alpha 24-77) CIA.
Discussion
Collagen vascular diseases such as Marfans and Loeys-Dietz syndrome affect the growth factor-beta pathway and are known to be associated with genetic risk factors that predispose patients to developing aortic aneurysms [5]. However, familial AAAsare genetically different and therefore pathologically different in that they are associated with late onset formation. Some studies have shown a link between 19q13 and 4q31 areas of the chromosome associated with aneurysm formation [6,7]. In one case report, Kitagawa et al showed anatomical similarities between monozygotic twins [8]; however, the pair of twins discussed here is dizygotic and had some anatomical differences as seen in Table 1. The variation may be due to the mode of inheritance of AAAs in which some families of AAA reveal a difference in penetrance patterns. Physiological effects such as hypertension, dyslipidemia and smoking may influence the architectural formation of the aneurysm and thus its variability between relatives. Furthermore, Darling et al. compared the architecture of AAAs and found no significant difference between familial and non-familial aneurysms anatomically or in occlusive burden [9].
When first degree relatives, defined as a parent, sibling or child develops aneurysmal degeneration, it is classified as a familial AAA [10]. This is an important classification from a screening perspective and can be established by history or physical exam of first-degree relatives.First-degree relatives are 2-3 times as likely to develop AAAs compared to the general population [3,11]. The current recommendations for the repair of AAAs are 5.5 cm for males and 5.0 cm for females [12]. However, familial AAAs has been documented to grow at an accelerated rate [13], with a higher rate of rupture [14].
In a Swedish study, a significant proportion of siblings of those with an AAA were found to have aneurysmal degeneration below the age of 65 years [15]. As such, the Canadian Society for Vascular Surgery recommends ultrasound surveillance on first-degree relatives after the age of 55 years [16]. As per the European Society for Vascular Surgery, those with a family history of AAAs are recommended to undergo ultrasound surveillance after 50 yearsof age, regardless of gender.
There is limited data on the efficacy of open versus endovascular repair of familial AAAs . Familial AAAs has an earlier onset, and as such, may be more amenable to open repair. However, Coscas et al. found that open repair was associated with late failure in patients with a positive family history of AAAs [17]. Conversely, patients undergoing endovascular repair experience higher rates of secondary sac rupture and higher re-intervention rates as compared to open repair [18].
Conclusion
Our case report demonstrates dizygotic twin brothers with architectural differences in the aneurysm diameter and length as well as thrombus burden. The presence of accessory renal arteries was also dissimilar; case 1 contained two accessory renal arteries on the left side and one on the right while the twin brother was found to have none. However, there were some similarities particularly in the circumferential position of the main renal arteries between cases. The overall dissimilarity in architecture may be due to genetic, developmental as well as life style factors between dizygotic twins.
REFERENCES
- Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605-2613.
- Thompson AR, Drenos F, Hafez H, Humphries SE. Candidate Gene Association Studies in Abdominal Aortic Aneurysm Disease: A Review and Meta-Analysis. Eur J Vasc Endovasc Surg. 2008;35:19-30.
- Kuivaniemi H, Kyo Y, Lenk G, Tromp G. Genome-wide approach to finding abdominal aortic aneurysm susceptibility genes in humans. In: Annals of the New York Academy of Sciences. 2006:270-281.
- Kuivaniemi H, Shibamura H, Arthur C, Berguer R, Cole CW, Juvonen T, et al. Familial abdominal aortic aneurysms: Collection of 233 multiplex families. J Vasc Surg. 2003;37:340-345.
- Gillis E, Van Laer L, Loeys BL. Genetics of thoracic aortic aneurysm: At the crossroad of transforming growth factor-ß signaling and vascular smooth muscle cell contractility. Circ Res. 2013;113:327-340.
- Shibamura H, Olson JM, Van Vlijmen-Van Keulen C, Buxbaum SG, Dudek DM, Tromp G, et al. Genome Scan for Familial Abdominal Aortic Aneurysm Using Sex and Family History as Covariates Suggests Genetic Heterogeneity and Identifies Linkage to Chromosome 19q13. Circulation. 2004;109:2103-2108.
- Darwood RJ, Brooks MJ. The impact of decreasing abdominal aortic aneurysm prevalence on a local aneurysm screening programme. Eur J Vasc Endovasc Surg. 2012;44:45-50.
- Kitagawa A, Komatsu H, Nagao T. Anatomical Similarity of Abdominal Aortic Aneurysms in Monozygotic Twins. Ann Vasc Dis. 2014;7:187-190.
- Darling RC, Brewster DC, Darling RC, LaMuraglia GM, Moncure AC, Cambria RP, et al. Are familial abdominal aortic aneurysms different? J Vasc Surg. 1989;10:39-43.
- van de Luijtgaarden KM, Bastos Gonçalves F, Hoeks SE, Valentijn TB, Stolker RJ, Majoor-Krakauer D, et al. Lower atherosclerotic burden in familial abdominal aortic aneurysm. J Vasc Surg. 2014;59:589-593.
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41.
- Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2.
- Akai A, Watanabe Y, Hoshina K, Obitsu Y, Deguchi J, Sato O, etal. Family history of aortic aneurysm is an independent risk factor for more rapid growth of small abdominal aortic aneurysms in Japan. J Vasc Surg. 2015;61:287-290.
- Sakalihasan N, Defraigne JO, Kerstenne MA, Cheramy-Bien JP, Smelser DT, Tromp G, et al. Family members of patients with abdominal aortic aneurysms are at increased risk for aneurysms: Analysis of 618 probands and their families from the liège AAA family study. Ann Vasc Surg. 2014;28:787-797.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg. 2012;56:305-310.
- Kapila V, Jetty P, Wooster D. The Canadian Society for Vascular Surgery 2018 Screening for abdominal aortic aneurysms in Canada: Review and position statement from the Canadian Society of Vascular Surgery. 2018.
- Coscas R, Greenberg RK, Mastracci TM, Eagleton M, Kang WC, Morales C, et al. Associated factors, timing, and technical aspects of late failure following open surgical aneurysm repairs. J Vasc Surg. 2010;52:272-281.
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374
Citation: Metias M, Patel A, Iyer V, Rapanos T (2021) Analysis of the Architectural Characteristics of the Abdominal Aortic Aneurysm in Dizygotic Twins. J Vasc Med Surg. 9:402
Copyright: © 2021 Metias M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

