Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research - (2023) Volume 15, Issue 4
An Open Label, Balanced, Randomized, Two Treatments, Two Sequences, Two Periods, Crossover, Single Dose, Oral Bioequivalence Study of Femelle 20 (Drospirenone 3 Mg and Ethinyl Estradiol 0.02 Mg) Coated Tablets of Abbott and Yaz® (Drospirenone 3 Mg And Ethinyl Estradiol 0.02 Mg) Coated Tablets of Bayer in Healthy, Adult, Human Female Subjects Under Fasting Condition
Arjun Arumugam O1*, Geethalakshmi G1, Srinivas G1, Kramm Karina2, Sasso Jaime2, Vergara Sebastian2, Ghünter Erich2, Salazar Boris2, Sosa María del Milagro2, Jimenez Julio2, Gavino-Gutiérrez Arquímedes M3, Lara Claudia4 and Higuera Maria Juliana52Abbott Laboratories Chile, Huechuraba, Chile
3Abbott Laboratories de Peru, Lima, Peru
4Abbott Laboratories de Mexico, Coyoacan, Mexico
5Abbott Laboratories de Colombia, Bogota, Colombia
Received: 31-Aug-2023, Manuscript No. JBB-23-22803; Editor assigned: 04-Sep-2023, Pre QC No. JBB-23-22803 (PQ); Reviewed: 18-Sep-2023, QC No. JBB-23-22803; Revised: 26-Sep-2023, Manuscript No. JBB-23-22803 (R); Published: 05-Oct-2023, DOI: 10.35248/0975-0851.23.15.528
Abstract
Yaz® Drospirenone and ethinyl estradiol is a Combined Oral Contraceptive (COC). Drospirenone is a spironolactone analogue with anti-mineralocorticoid and anti-androgenic activity. The estrogen in Yaz® is ethinyl estradiol. COCs lower the risk of becoming pregnant primarily by suppressing ovulation. Yaz® is also indicated for the treatment of symptoms of Pre Menstrual Dysphoric Disorder (PMDD) in women who choose to use an oral contraceptive as their method of contraception. The purpose of this study was to evaluate the bioequivalence between Femelle 20 (Drospirenone 3 mg and ethinyl estradiol 0.02 mg) coated tablets of Abbott versus Yaz® (Drospirenone 3 mg and ethinyl estradiol 0.02 mg) coated tablets of Bayer under fasting condition in healthy non-pregnant female subjects. An open label, balanced, randomized, two treatments, two sequences, two periods, crossover, single dose study with washout period of 14 days was carried out in 36 healthy non-pregnant female subjects in the age group of 22 to 42 years, who met the study eligibility criteria, participated in the study and all 36 subjects completed both the periods of the study. The pharmacokinetic samples collected from subjects who completed the study were analyzed to determine the plasma concentration of Drospirenone and ethinyl estradiol using a validated bio-analytical method. Ninety percent confidence interval of Cmax and AUC0-72 were 100.45%-119.63% and 98.04%-104.50%, respectively for Drospirenone and 90% confidence interval of Cmax, AUC0-t and AUC0-∞ were 93.76%-102.39%, 102.42%-110.02% and 101.37%-108.84%, respectively for ethinyl estradiol, which were within the acceptable limits of 80.00% to 125.00%.Keywords
Drospirenone; Ethinyl estradiol; Bioavailability; Bioequivalence; Pharmacokinetic; Combined oral contraceptive
Abbrevations
AUC: Area under the concentration versus time curve; AUC0-t: Area under the plasma concentration versus time curve from zero to time t; AUC0-72: Area under the plasma concentration versus time curve from zero to 72; BMI: Body Mass Index; CDSCO: Central Drugs Standard Control Organization; Cmax: Concentration Maximum; CV: Coefficient of Variation; IEC: Independent Ethics Committee; ISCV: Intra-subject Co-efficient of Variation; LCMS/ MS: Liquid Chromatography Tandem Mass Spectrometry; mg: Milligram; mL: Millilitre; mM: Milli Molar; ng/mL: Nanogram per millilitre; PK: Pharmacokinetic; Tmax: Time taken to reach maximum concentration; COC: Combined Oral Contraceptive; PMDD: Premenstrual Dysphoric Disorder
Introduction
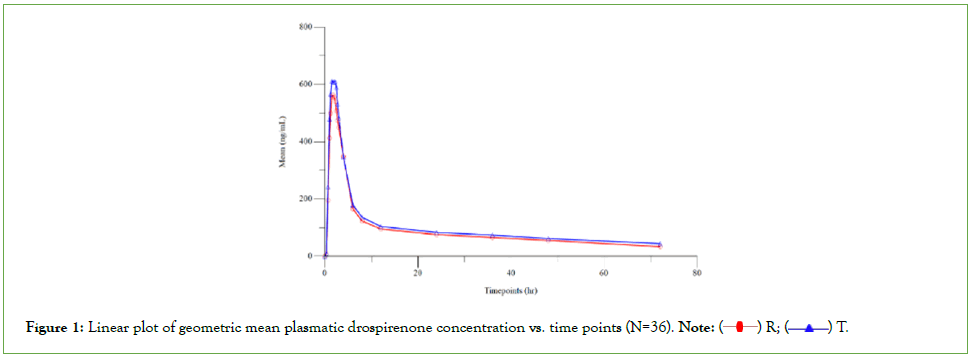
Contraceptives are most effective means for contraception excluding sterilization. Contraceptives are hormonal agents; combination oral contraceptives contain both an estrogen and a progestogen. Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Drospirenone is a synthetic progestin and spironolactone analog with anti-mineralocorticoid activity. The primary estrogen used in oral contraceptives is ethinylestradiol. 17-Ethinyl Estradiol (EE), a synthetic estrogen developed in 1938, is an essential constituent of oral contraceptives, which have been widely prescribed since the 1970s. In general, ethinylestradiol is used in combination to prevent pregnancy in women. Drospirenone is a novel synthetic progestogen with a pharmacological profile similar to that of natural progesterone [1,2]. The compound is part of certain birth control formulations. Combined with ethinyl estradiol in oral contraceptive formulations, drospirenonecontaining contraceptives have similar efficacy and safety profiles to other low-dose oral contraceptives, but seem to offer improved tolerability with regard to weight gain, mood changes, acne and treatment of a severe form of the premenstrual syndrome called premenstrual dysphoric disorder. Drospirenone (6R,7R,8R,9S,10 R,13S,14S,15S,16S,17S)-1,3’,4’,6,6a,7,8,9,10,11, 12,13,14,15,15a,16- hexadecahydro-10,13-dimethylspiro-[17H-dicyclopropa cyclopenta [a] phenanthrene-17,2’(5H)-furan]-3,5’(2H)-dione)} is a synthetic progestational compound and has a molecular weight of 366.5 and a molecular formula of C24H30O3. ethinyl estradiol (19-nor- 17α-pregna 1, 3, 5(10)-triene-20-yne-3, 17-diol) is a synthetic estrogenic compound and has a molecular weight of 296.4 and a molecular formula of C20H24O2. The aim of this study was to compare in healthy volunteers, the pharmacokinetics profiles and evaluate the bioequivalence of Femelle 20 (Drospirenone 3 mg and ethinyl estradiol 0.02 mg) coated tablets of Abbott versus Yaz® (Drospirenone 3 mg and ethinyl estradiol 0.02 mg) coated tablets of Bayer (Figure 1) [3-6].
Figure 1: Linear plot of geometric mean plasmatic drospirenone concentration vs. time points (N=36). Note:  R;
R;  T.
T.
Materials and Methods
Test product, dose and mode of administration, Lot
Femelle® 20 (Drospirenone 3 mg and ethinyl estradiol 0.02 mg) Coated Tablets, 02 × 3 mg/0.02 mg, Oral with 200 mL of water in sitting posture under fasting condition, C200375.
Test product, dose and mode of administration, Lot
Femelle® 20 (Drospirenone 3 mg and ethinyl estradiol 0.02 mg)
product, dose and mode of administration, Lot
Yaz® (Drospirenone 3 mg and ethinyl estradiol 0.02 mg) Coated Tablets, 02 × 3 mg/0.02 mg, Oral with 200 mL of water in sitting posture under fasting condition, BS01R33 (Tables 1 and 2).
| Parameters | Antilog least square mean | T/R ratio (%) | 90% Confidence interval | Intra subject CV (%) | Power | |
|---|---|---|---|---|---|---|
| Test product (T) | Reference product (R) | |||||
| Ln (Cmax) | 95.13 | 86.7817 | 109.62 | 100.45%-119.63% | 22.18 | 0.9936 |
| Ln (AUC0-72) | 1218.7038 | 1204.0252 | 101.22 | 98.04%-104.5% | 8.01 | 1 |
Table 1: Statistical results of test product-T versus reference product-R for drospirenone (N=36).
| Parameters | Antilog least square mean | T/R ratio (%) | 90% Confidence interval | Intra subject CV (%) | Power | |
|---|---|---|---|---|---|---|
| Test product (T) | Reference product (R) | |||||
| Ln (Cmax) | 79.2937 | 80.9261 | 97.98 | 93.76%-102.39% | 10.75 | 1 |
| Ln (AUC0-t) | 821.7171 | 774.0676 | 106.16 | 102.42%-110.02% | 8.73 | 1 |
| Ln (AUC0-∞) | 997.4058 | 949.5713 | 105.04 | 101.37%-108.84% | 8.67 | 1 |
Table 2: Statistical results of test product-T versus reference product-R for ethinyl estradiol (N=34).
Methodology
All The study protocol was prepared and IEC approval was obtained before initiation of the study. Study subjects were screened and enrolled in the study as per the IEC approved protocol. Written informed consent was obtained from each volunteer for screening prior to initiation of screening procedure and for the study prior to enrolment. Individual counseling was then given to the willing volunteers by the Investigator in private and any questions and concerns were addressed prior to obtaining consent. The Principal investigator/sub-investigator/physician reviewed all the screening results to assess eligibility of each volunteer. Subjects were enrolled in the study based on the inclusion and exclusion criteria. This study was designed based on the known pharmacokinetic profile of the investigational product and general accepted standards for the conduct of bioequivalence study. Thirty-six healthy nonpregnant female subjects in the age group of 22 to 42 years, who met the study eligibility criteria, participated in the study and all 36 subjects completed both the periods of the study. The clinical study was conducted over a period of 38 days. Blood sampling was done at pre-defined intervals up to 72.00 hours in both periods, separated by a washout period of 14 days between each period. The plasma concentrations of Drospirenone and ethinyl estradiol were quantified using a validated method in LC-MS/MS method in samples collected from all 36 study subjects that complete the study. However, for ethinyl estradiol, concentrations of only 34 subjects were reported following bio-analysis; since for two subjects, concentrations were reported as “Not Sufficient (NS)” owing to insufficient quantity of plasma sample to perform repeat analysis after failure of initial analysis to meet the acceptance criteria per the in-house SOP. The pharmacokinetic and statistical analysis of Drospirenone and ethinyl estradiol were performed using the concentration data obtained from 36 and 34 subjects, respectively. Drospirenone and ethinyl estradiol, Analysis of Variance (ANOVA) was performed on the Ln-transformed data of Cmax and AUC0-72 and Cmax, AUC0-t and AUC0-∞, respectively using PROC GLM of SAS® version 9.4 (Figures 2) [7].
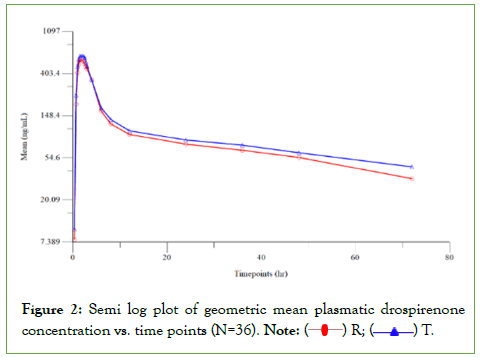
Figure 2: Semi log plot of geometric mean plasmatic drospirenone concentration vs. time points (N=36). Note:  R;
R;  T.
T.
Study criteria for inclusion/exclusion of subjects
Healthy non-pregnant, non-breast feeding female literate volunteers of 18 to 45 years (both years inclusive) with BMI of 18.50-30.00 Kg/ m2 and weight >50 Kg, were eligible to be enrolled in the study.
Inclusion criteria encompassed no evidence of cardiac, pulmonary, gastrointestinal, hepatic, renal, hematologic, or neurologic disorders, or any acute or chronic disease, no history of drug or alcohol addiction, normal laboratory tests (complete blood counts, urinalysis, liver and kidney function, serum pregnancy test and blood sugar); and serological negativity for HIV and hepatitis B. Subjects were informed by an investigator about the purposes and risks of the study. They were asked to refrain from using concomitant medications, including over-the-counter products, dietary supplements and natural products which potentially modify kinetics/dynamics of Drospirenone and ethinyl estradiol, 14 days prior to dosing and throughout the end of the study. Consumption of grapefruit and/or its products were not allowed within 10 days prior to the start of the study. Caffeine and/or xanthine-containing products or alcohol were not allowed 48 hours prior the first administration of the study medications and throughout the blood sampling periods (Tables 3-5) [8].
| Parameter | Mean | SD | Min | Max |
|---|---|---|---|---|
| Age (years) | 35 | 5 | 22 | 42 |
| Height (m) | 1.544 | 0.06 | 1.405 | 1.675 |
| Weight (Kg) | 63.1 | 7.5 | 50 | 80.7 |
| BMI (Kg/m2) | 26.47 | 2.62 | 21.52 | 32.13 |
Table 3: Summarized demographic profile of study completers (N=36).
| Parameters | N | Reference (R) Mean ± SD |
|---|---|---|
| Cmax (ng/ml) | 36 | 90.069 ± 23.626 |
| AUC0-72 (ng.hr/ml) | 36 | 1229.741 ± 256.709 |
| *Tmax (hr) | 36 | 1.75 (0.75-4.50) |
Note: Expressed in terms of median (range)
Table 4: Summary of pharmacokinetic parameters for drospirenone of reference.
| Parameters | N | Test (T) Mean ± SD |
|---|---|---|
| Cmax (ng/mL) | 36 | 98.132 ± 25.471 |
| AUC0-72 (ng.hr/mL) | 36 | 1242.059 ± 239.068 |
| *Tmax (hr) | 36 | 1.50 (0.50-4.00) |
Note: Expressed in terms of median (range)
Table 5: Summary of pharmacokinetic parameters for drospirenone of test product.
Sample size and power
Blood For an expected mean difference of 5% between the formulations, an expected intra-subject CV of around 22% for Cmax and 10.27% for AUC, 30 subjects would be required to prove bioequivalence at more than 0.90 of power. Based on crossover design and considering possible dropouts and withdrawals, a sample size of 36 subjects was considered to establish bioequivalence between formulations (Figure 3).
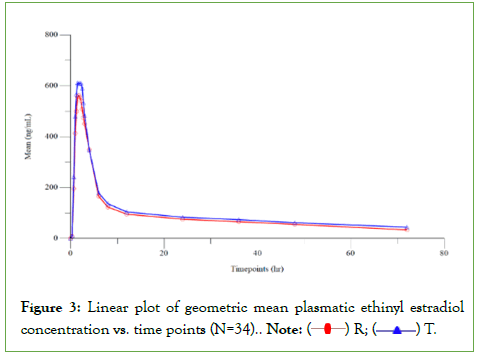
Figure 3: Linear plot of geometric mean plasmatic ethinyl estradiol concentration vs. time points (N=34). Note:  R;
R;  T.
T.
Subjects drug administration and blood sampling
After an overnight fasting of 10 hours, subjects were administered with a single oral dose (02 tablets as single dose) of Test product or Reference product with 200 mL of water as per the randomization schedule in sitting posture in each period. Compliance to drug administration was assessed by examination of the oral cavity and hands of the subject immediately after dosing. All the subjects remained in sitting posture for 02 hours after dosing except one subject, who did not maintain posture restriction for the management of an adverse event. During this restriction period, the subjects were permitted to walk for reasons such as but not limited to the following: natural exigencies. Subjects were restricted from consumption of water for 01 hour before and 01 hour after dosing in each period and were allowed to drink water ad libitum thereafter. Washout period of 14 days was maintained between the treatments. The pharmacokinetic profile (in terms of rate and extent of absorption) of both test and reference products was evaluated based on measured concentration of drug in the human plasma samples collected during the clinical phase. Blood samples for pharmacokinetic analysis were designed appropriately for characterizing the pharmacokinetic profile for the given treatments at the dose administered. Blood samples of 05 mL each were collected at 00.00 hour (pre-dose), 00.25, 00.50, 00.75, 01.00, 01.50, 02.00, 02.50, 03.00, 03.50, 04.00, 04.50, 05.00, 06.00, 08.00, 10.00, 12.00, 16.00, 24.00, 48.00 and 72.00 hours post-dose for measurement of pharmacokinetic parameters of Drospirenone and ethinyl estradiol in both the periods (Table 6).
| Parameter | N | Reference (R) (Mean ± SD) |
|---|---|---|
| Cmax (pg/mL) | 34 | 84.077 ± 23.373 |
| AUC0-t (pg.hr/mL) | 34 | 839.438 ± 354.445 |
| AUC0-∞ (pg.hr/mL) | 34 | 1011.528 ± 378.181 |
| *Tmax (hr) | 34 | 1.50 (0.75 - 4.50) |
| Kel (hr-1) | 34 | 0.042 ± 0.013 |
| T1/2 (hr) | 34 | 18.105 ± 5.017 |
| VD | 34 | 537532.189 ± 101333.446 |
| CL | 34 | 22347.514 ± 7587.162 |
Table 6: Summary of pharmacokinetic parameters for ethinyl estradiol of reference product-(R).
Results and Discussion
Tolerability
Subjects were monitored for adverse events during the study. Subjects were instructed to inform clinic personnel of any untoward medical symptoms and/or events that arose during the course of the study. The Principal Investigator/sub-investigator/study physician also evaluated the subjects for subsequent dosing. Each adverse event reported by the subjects during the study was assessed for its severity, relationship with the study drug and outcome of the adverse event. Safety was assessed from the screening period to the end of the study through clinical examinations, vital signs assessment, 12-lead Electro Cardio Gram (ECG), clinical laboratory parameters (e.g. Hematology, Biochemistry, Urine analysis and Serology test) and monitoring subjects’ well-being, symptoms and signs for adverse events. No serious adverse events were reported during the conduct of this study. A total of 11 non-serious adverse events were reported by 08 subjects during the study. Five adverse events were reported following administration of the test product and six adverse events with reference product. Out of the 11 nonserious adverse events reported in the study 08 events (Vomiting) were evaluated to be “Probably” related and were expected following exposure to the drug product. Three (03) events were evaluated to be “Unlikely” in relation and were unexpected with the study drug. Subjects were followed up by the investigator/physician and all non-serious adverse events resolved without any sequelae. Thus, it could be considered that both the test and reference products are well tolerated by the study subjects at the selected dose level. The incidence of AEs in the study for reference product was (16.66%) and test product was (13.88%) (Table 7).
| Parameter | N | Test (T) (Mean ± SD) |
|---|---|---|
| Cmax (pg/mL) | 34 | 82.473 ± 23.100 |
| AUC0-t (pg.hr/mL) | 34 | 895.244 ± 386.234 |
| AUC0-∞ (pg.hr/mL) | 34 | 1068.771 ± 414.642 |
| *Tmax (hr) | 34 | 2.00 (1.00 - 5.00) |
| Kel (hr-1) | 34 | 0.044 ± 0.018 |
| T1/2 (hr) | 34 | 17.516 ± 5.361 |
| VD | 34 | 489037.577 ± 91188.849 |
| CL | 34 | 21439.758 ± 7954.369 |
Note: Expressed in terms of median (range)
Table 7: Summary of pharmacokinetic parameters for ethinyl estradiol of test.
Pharmacokinetic and statistical analysis
A total of 36 subjects were enrolled and all the 36 subjects completed the study. Thus, 36 pharmacokinetic samples collected were analyzed for determining the concentrations of Drospirenone and ethinyl estradiol in plasma. The drug concentrations reported for 36 subjects following bio-analysis of Drospirenone were included for pharmacokinetic analysis and concentrations reported for 34 subjects following bio-analysis of ethinyl estradiol were included for pharmacokinetic analysis, excluding data of two subjects, for whom the concentrations were reported as “Not Sufficient (NS)” owing to the insufficient quantity of plasma samples to perform a repeat analysis, as the initial batch failed to meet the acceptance criteria per the in-house SOP. Pharmacokinetic parameters were calculated using Phoenix® WinNonlin® software version 8.1. The mean, standard deviation, standard error, geometric mean, coefficient of variation, minimum, median, maximum and range were calculassted for Cmax, AUC0-72 and Tmax for Drospirenone and Cmax, AUC0-t, AUC0-∞, Tmax, VD, CL, t ½ and Kel for ethinyl estradiol [9].
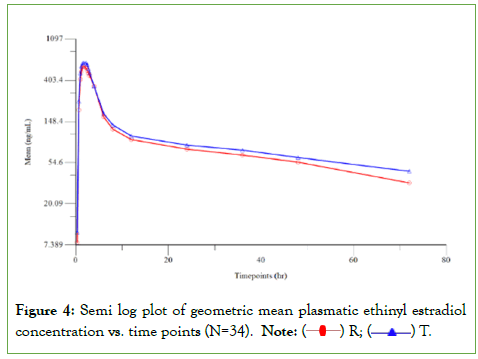
Thirty-six healthy non-pregnant female subjects in the age group of 22 to 42 years, who met the study eligibility criteria, participated in the study and all 36 subjects completed both the periods of the study. The clinical study was conducted over a period of 38 days. Blood sampling was done at pre-defined intervals up to 72.00 hours in both the periods, separated by a washout period of 14 days between each period. The plasma concentrations of Drospirenone and ethinyl estradiol were quantified using a validated method in LC-MS/MS method in samples collected from all 36 study completers. However, for ethinyl estradiol, concentrations of only 34 subjects were reported following bio-analysis; since for two subjects, concentrations were reported as “Not Sufficient (NS)” owing to insufficient quantity of plasma sample to perform repeat analysis after failure of initial analysis to meet the acceptance criteria per the in-house SOP. The pharmacokinetic and statistical analysis of Drospirenone and ethinyl estradiol were performed using the concentration data obtained from 36 and 34 subjects, respectively. Ninety percent confidence interval of Cmax and AUC0- 72 were 100.45%-119.63% and 98.04%-104.50% for Drospirenone and Cmax, AUC0-t and AUC0-∞ were 93.76%-102.39%, 102.42%- 110.02% and 101.37%-108.84% for ethinyl estradiol, which were within the acceptable limits of 80.00% to 125.00% (Figure 4) (Tables 8 and 9) [10].
Figurce 4: Semi log plot of geometric mean plasmatic ethinyl estradiol concentration vs. time points (N=34). Note:  R;
R;  T.
T.
| Parameters | Cmax | AUC0-72 | Significance |
|---|---|---|---|
| Sequence effect | 0.5069 | 0.9781 | Insignificant for Cmax and AUC0-72 |
| Period effect | 0.0758 | 0.0302 | Insignificant for Cmax and significant for AUC0-72 |
| Treatment (Formulation) effect | 0.0843 | 0.5246 | Insignificant for Cmax and AUC0-72 |
| Subjects nested within sequence effect | 0.0237 | <0.0001 | Significant for Cmax and AUC0-72 |
Note: p<0.10 for sequence effect and p<0.05 for all other effects considered to be significant.
Table 8: p-value for Cmax and AUC of drospirenone.
| Parameters | Cmax | AUC0-t | AUC0-∞ | Significance |
|---|---|---|---|---|
| Sequence effect | 0.1225 | 0.1247 | 0.1471 | Insignificant for Cmax, AUC0-t and AUC0-∞ |
| Period effect | 0.7662 | 0.0796 | 0.2091 | Insignificant for Cmax, AUC0-t and AUC0-∞ |
| Treatment (Formulation) effect | 0.439 | 0.008 | 0.0256 | Insignificant for Cmax and significant for AUC0-t and AUC 0-∞ |
| Subjects nested within sequence effect | <0.0001 | <0.0001 | <0.0001 | Significant for Cmax, AUC0-t and AUC0-∞ |
Note: p<0.10 for sequence effect and p<0.05 for all other effects considered to be significant.
Table 9: p-value for Cmax and AUC of ethinylestradiol
Conclusion
Bioequivalence was demonstrated between Femelle® 20 (Drospirenone 3 mg and ethinyl estradiol 0.02 mg) coated tablets of Abbott and Yaz® (Drospirenone 3 mg and ethinyl estradiol 0.02 mg) coated tablets of Bayer in healthy, adult, human female subjects under fasting condition. The 90% CI of Drospirenone and ethinyl estradiol were within the acceptable limits of 80.00 to 125.00%. Based on the adverse events and proceeds of the study it can be concluded that the study medications were relatively well tolerated by the study subjects at selected dose level.
Acknowledgement
This scientific article was made with funding from Abbott, whose participation was related to financial support and document review. All technical, clinical and analytical execution of the bioequivalence studies were performed independently by Azidus Laboratories LTD.
References
- Integrated addendum to ich e6(r1): Guideline for good clinical practice. GCP. 2016.
[Google Scholar] [PubMed]
- Lu DY.CDSCOâ??s New Drugs and Clinical Trials Rules.G.S.R. 2019.227(E).
- Structure and Content of Clinical Study Reports E3. Current Step 4 version. 1995.
- 21 Code of Federal Regulations. USFDA. 2023.
- Guide for the performance of comparative bio-availability studies in solid pharmaceutical forms of oral administration and systemic action. Pharmaceutics. 2016;13(3):363-364.
- Prescribing Information of Yaz® (Drospirenone 3 mg and Ethinyl Estradiol 0.02 mg) coated tablets of Bayer. HealthCare Pharmaceuticals Inc.2001.
- Draft product specific guidance on Drospirenone and Ethinyl Estradiol. FDA.2013.
- Bioequivalence of of Two Oral Contraceptive Drugs Containing Ethinylestradiol and Drospirenone in Healthy Female Volunteers. State University of Campinas.1989;40(5):581-590.
[Crossref] [Google Scholar] [PubMed]
- Keam S J. Ethinylestradiol/drospirenone: A review of its use as an oral contraceptive. Treat. Endocrinol. 2003;2(1): 49-70.
[Crossref] [Google Scholar] [PubMed]
- Huber J. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. Eur. J. Contracept. Reprod. Health Care.2000;5(2):25-34.
[Crossref] [Google Scholar] [PubMed]
Citation: Arumugam OA, Lakshmi GG, Gopineedu S, Karina K, Jaime S, Sebastian V, et al. (2023) An Open Label, Balanced, Randomized, Two Treatments, Two Sequences, Two Periods, Crossover, Single Dose, Oral Bioequivalence Study of Femelle 20 (Drospirenone 3 Mg and ethinyl estradiol 0.02 Mg) Coated Tablets of Abbott and Yaz® (Drospirenone 3 Mg And ethinyl estradiol 0.02 Mg) Coated Tablets of Bayer in Healthy, Adult, Human Female Subjects Under Fasting Condition. J Bioequiv Availab. 15:528.
Copyright: ©2023 Arumugam OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.