Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- CiteFactor
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 15, Issue 5
An Open Label, Balanced, Randomized, Two Treatments, Two Sequences, Two Periods, Crossover, Single Dose, Bioequivalence Study of Olmesartan medoxomil/Hydrochlorothiazide 20 mg/12.5 mg Coated Tablets of Abbott and Olmetec plus® (Olmesartan medoxomil/Hydrochlorothiazide) 20 mg/12.5 mg Coated Tablets of Daiichi-Sankyo in Healthy, Adult, Human Subjects Under Fasting Condition
Arjun Arumugam O1*, Geethalakshmi G1, Srinivas G1, Hurtado-Colorado Karen2, Higuera Maria Juliana2, Gavino-Gutiérrez Arquímedes M3, Lara Claudia4, Cereceda Gabriel5, Leon Isaac5, Tagle Arturo Ruiz5, Gonzalez Camila5, Pinto Deny5, Echeverria Patricia5, Lobos María Francisca5, Calderon Sebastián5, Sosa María del Milagro5, Sasso Jaime5, Kramm Karina5 and Jimenez Julio52Abbott Laboratories de Colombia, Bogota, Colombia
3Abbott Laboratories de Peru, Lima, Peru
4Abbott Laboratories de Mexico, Coyoacan, Mexico
5Abbott Laboratories Chile, Huechuraba, Chile
Received: 11-Sep-2023, Manuscript No. JBB-23-22801; Editor assigned: 15-Oct-2023, Pre QC No. JBB-23-22801 (PQ); Reviewed: 29-Oct-2023, QC No. JBB-23-22801; Revised: 06-Nov-2023, Manuscript No. JBB-23-22801 (R); Published: 13-Oct-2023, DOI: 10.35248/0975-0851.23.15.538
Abstract
Olmesartan Medoxomil and Hydrochlorothiazide is a combination of an Angiotensin II receptor antagonist (olmesartan medoxomil) Thiazide diuretic (Hydrochlorothiazide) used in the treatment of mild to moderate essential hypertension in patients who requires combination therapy. The purpose of this study was to evaluate the bioequivalence between Olmesartan Medoxomil/Hydrochlorothiazide 20 mg/12.5 mg coated tablets of Abbott and Olmetec Plus® (Olmesarten Medoxomil/Hydrochlorothiazide) 20 mg/12.5 mg coated tablets of Daiichi-Sankyo in healthy, adult, human subjects. An open label, balanced, randomized two treatments, two sequences, two periods, crossover, single dose study with washout period of 02 days under fasting condition was carried out in 28 subjects in the age group of 20 to 44 years and 27 subjects completed the study. All the subjects included in the study were males and Asians. The pharmacokinetic samples collected from subjects who completed the study were analysed to determine the plasma concentration of Olmesartan and Hydrochlorothiazide using a validated bio-analytical method. Hydrochlorothiazide is the 90% confidence interval of the relative mean Cmax, AUC0-t and AUC0-∞ of Hydrochlorothiazide were 94.40%-107.65%, 94.97%-105.83%, 95.00%-105.67% respectively, which were within the acceptable limits of 80.00 to 125.00%. Olmesartan: The 90% confidence interval of Cmax, AUC0-t and AUC0-∞ for Olmesartan were 92.31%-107.60%, 91.81%-104.11% and 92.76%-104.56% respectively, which were within the acceptable limits of 80.00% to 125.00%.
Keywords
Olmesartan; Hydrochlorothiazide; Bioavailability; Bioequivalence; Pharmacokinetic
Abbrevations
AUC: Area Under the Concentration versus time curve; AUC0-t: Area Under the plasma Concentration versus time curve from zero to time at which last measurable concentration; AUC0-∞: Area Under the plasma Concentration versus time curve, from zero to infinity; BMI: Body Mass Index; Cmax :Concentration Maximum; CV: Coefficient of Variation; ISCV : Intra-subject Co-efficient of Variation; LCMS/MS: Liquid Chromatography Tandem Mass Spectrometry; mg: Milligram; mL: Millilitre; ng/mL: Nanogram per millilitre; PK: Pharmacokinetic; Kel : Elimination constant; T½: Half-life; Tmax : Time taken to reach maximum concentration; IEC :Independent Ethics Committee
Introduction
In the quest to provide the patients with affordable and effective medicines, many drug manufacturers intend manufacturing a copy of the innovator drugs which are usually very expensive. These are termed as generic drugs. To ensure that only the safe and effective alternatives are reached to the market, the regulatory agencies like United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), ANVISA, INVIMA, ISP, Chile and few other countries had postulated few sets of guidelines specific to their agency. To ensure that the alternative treatment available in the market is as efficacious as the innovator product, the requirement of bioequivalence evolved. A bioequivalence study has become a mandatory requirement for the manufacturer to obtain a marketing authorization approval from the health agency. Olmesartan Medoxomil and Hydrochlorothiazide is a combination of an Angiotensin II receptor antagonist (olmesartan medoxomil) Thiazide diuretic (Hydrochlorothiazide) [1,2].
Olmesartan medoxomil
The chemical name is 2,3-dihydroxy-2-butenyl-4-(1-hydroxy-lmethylethyl)- 2-propyl-1-[p(o-lH-tetrazol-5-ylphenyl)benzyl]imidazole- 5-carboxylate, cyclic-2,3-carbonate, its molecular formula is C29H30N6O6, and its molecular weight is 558.6. Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder. It is insoluble in water and sparingly soluble in methanol.
Hydrochlorothiazide
The chemical name is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine- 7-sulfonatnide1,1-dioxide. Its molecular formula is C7H8CIN3O4S2, Hydrochlorothiazide is a white or practically white, crystalline powder. It is slightly soluble in water but freely soluble in sodium hydroxide solution. This study was designed to evaluate the relative bioavailability of the test product Olmesartan Medoxomil/ Hydrochlorothiazide 20 mg/12.5 mg Coated Tablets of Abbott and reference product Olmetec Plus® (Olmesartan medoxomil/ Hydrochlorothiazide) 20 mg/12.5 mg Coated Tablets of Daiichi- Sankyo in healthy, adult, human subjects under fasting condition. (Figure 1) [3-5].
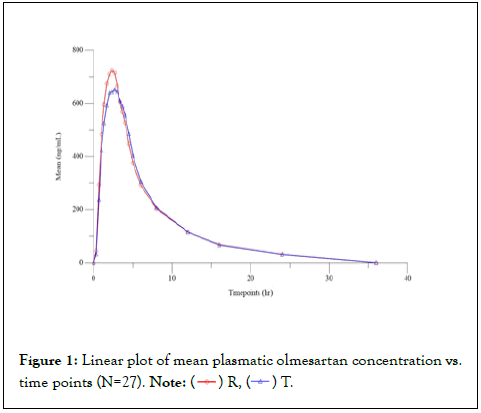
Figure 1: Linear plot of mean plasmatic olmesartan concentration vs.
time points (N=27). Note: ( ) R, (
) R, ( ) T.
) T.
Materials and Methods
Test product, dose and mode of administration
Olmesartan medoxomil/Hydrochlorothiazide Coated Tablets, 01 × 20 mg/12.5 mg, Oral with 200 mL of water in sitting posture under fasting condition, 21ID112.
Reference product, dose and mode of administration
Olmetec Plus® 20 mg/12.5 mg (Olmesartan Medoxomil/ Hydrochlorothiazide) flim-coated tablets, 01 × 20 mg/12.5 mg, Oral with 200 mL of water in sitting posture under fasting condition (Tables 1 and 2).
| Parameters | Antilog least square mean | Point estimate (%) | 90% Confidence Interval | ISCV (%) | Power | |
|---|---|---|---|---|---|---|
| Test product (T) | Reference product (R) | |||||
| Ln (Cmax ) | 753.8763 | 756.436 | 99.66 | 92.31-107.60 | 16.58 | 0.9984 |
| Ln (AUC0-t) | 4535.474 | 4638.9966 | 97.77 | 91.81-104.11 | 13.58 | 0.9999 |
| Ln (AUC0--∞) | 4891.2068 | 4966.4524 | 98.48 | 92.76-104.56 | 12.93 | 1 |
Table 1: Statistical results of test product-T vs. reference product-R for olmesartan.
| Parameters | Antilog least square mean | Point estimate (%) | 90% Confidence Interval | ISCV (%) | Power | |
|---|---|---|---|---|---|---|
| Test product (T) | Reference product (R) | |||||
| Ln (Cmax ) | 86.3683 | 85.6784 | 100.81 | 94.40-107.65 | 14.18 | 0.9998 |
| Ln (AUC0-t) | 609.3233 | 607.797 | 100.25 | 94.97-105.83 | 11.67 | 1 |
| Ln (AUC0--∞) | 640.7408 | 639.4957 | 100.19 | 95.00-105.67 | 11.48 | 1 |
Table 2: Statistical results of test product-T vs. reference product-R for hydrochlorothiazide.
Methodology
The study protocol with annexes was prepared and IEC approval was obtained before initiation of the study. The subjects were screened and enrolled in the study as per the IEC approved protocol. Written informed consent was obtained from each volunteer in screening visit to initiation of screening procedure and for the study prior to enrolment. Individual counselling was then given to the willing volunteers by the Investigator in private and any questions and concerns were addressed prior to obtaining consent. The principal investigator/sub-investigator/physician reviewed all the screening results to assess eligibility of each volunteer. Subjects were enrolled in the study based on the inclusion and exclusion criteria. This study was designed based on the known pharmacokinetic profile of the investigational product and general accepted standards for the conduct of bio-equivalence study.
28 subjects who met the eligibility criteria were enrolled and dosed with a single dose of either test or reference product in sitting posture at a fixed time in each period. Washout period of 02 days was maintained between each period, in order to minimize any possibility of carryover effect from the preceding treatment. Blood samples were collected at pre-defined time intervals for the measurement of concentration and pharmacokinetic parameters of Olmesartan and Hydrochlorothiazide in both the periods. Data obtained from 27 subjects who completed the study were used for the pharmacokinetic and statistical analysis of Olmesartan and Hydrochlorothiazide. Bioequivalence was determined by statistical comparison of Ln-transformed data of Cmax, AUC0-t and AUC0-∞ of the test and reference formulations using SAS version 9.4. (Figures 2) [6].
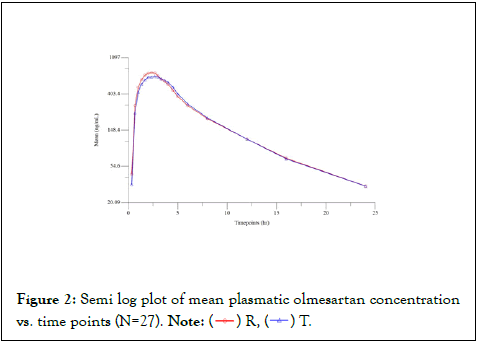
Figure 2: Semi log plot of mean plasmatic olmesartan concentration vs. time points (N=27).Note: ( ) R, (
) R, ( ) T.
) T.
Study criteria for inclusion/exclusion of subjects
Healthy volunteers aged 18 to 45 years, and with BMI of 18.50- 29.99 Kg/m2 and weight>50 Kg were eligible to be enrolled in the study. Inclusion criteria encompassed no evidence of cardiac, pulmonary, gastrointestinal, hepatic, renal, hematologic, or neurologic disorders, or any acute or chronic disease, no history of drug or alcohol addiction, normal laboratory tests (complete blood counts, urinalysis, liver and kidney function, and blood sugar); and serological negativity for HIV and hepatitis B. Subjects were informed by an investigator about the purposes and risks of the study.
They were asked to refrain from using concomitant medications, including over-the-counter counter products, dietary supplements and natural products which potentially modify kinetics/dynamics of Olmesartan and Hydrochlorothiazide, 14 days prior to dosing and throughout the end of the study. Consumption of grapefruit and/or its products were not allowed within 10 days prior to the start of the study. Caffeine and/or xanthine-containing products or alcohol were not allowed 48 hours prior the first administration of the study medications and throughout the blood sampling periods (Tables 3-5) [7].
| Parameter | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 32 | 6 | 20 | 44 |
| Height (m) | 1.694 | 0.047 | 1.62 | 1.78 |
| Weight (kg) | 68.9 | 9.2 | 54.3 | 88.6 |
| BMI (kg/m2) | 23.96 | 2.77 | 18.92 | 29 |
Table 3: Summarized demographic profile of subjects who completed the study (N=27).
| Parameter | N | Reference(R) (Mean ± SD) |
|---|---|---|
| Cmax (ng/mL) | 27 | 783.304 ± 190.695 |
| AUC0-t (hr.ng/mL) | 27 | 4778.665 ± 1170.192 |
| AUC0-∞ (hr.ng/mL) | 27 | 5108.964 ± 1220.097 |
| AUC% Extrap_Obs | 27 | 6.582 ± 1.668 |
| *Tmax (hr) | 27 | 2.33 (1.33- 5.00) |
| T1/2 (hr) | 27 | 6.085 ± 1.081 |
| Kel (hr-1) | 27 | 0.118 ± 0.023 |
| VD (mL) | 27 | 36064.438 ± 9597.529 |
| CL (mL/hr) | 27 | 4156.376 ± 1099.068 |
Note: *Expressed in terms of median (range)
Table 4: Summary of pharmacokinetic parameters for olmesartan of reference product-R.
| Parameter | N | Reference(R) (Mean ± SD) |
|---|---|---|
| Cmax (ng/mL) | 27 | 777.300 ± 195.372 |
| AUC0-t (hr.ng/mL) | 27 | 4681.526 ± 1216.248 |
| AUC0-∞ (hr.ng/mL) | 27 | 5035.529 ± 1260.588 |
| AUC%Extrap_Obs | 27 | 7.239 ± 2.043 |
| *Tmax (hr) | 27 | 2.05 (1.33-4.50) |
| T1/2 (hr) | 27 | 6.286 ± 1.243 |
| Kel (hr-1) | 27 | 0.115 ± 0.024 |
| VD (mL) | 27 | 38093.235 ± 11930.753 |
| CL (mL/hr) | 27 | 4218.347 ± 1082.812 |
Note: *Expressed in terms of median (range)
Table 5: Summary of pharmacokinetic parameters for olmesartan of test product-T
Sample size and power
For an expected mean difference of 5% between the formulations, with an expected intrasubject CV: Olmesartan (16.5% for Cmax and 16.3% for AUC) Hydrochlorothiazide (18.1% for Cmax and 12.8% for AUC). Therefore, 21 subjects were required to prove bioequivalence at 0.90 powers. Based on crossover design and considering possible dropouts due to expected adverse drug reaction (ADR), a sample size of 28 subjects were sufficient to establish bioequivalence between formulations. ISCV of Cmax of hydrochlorothiazide was considered for sample size calculation (Figure 3).
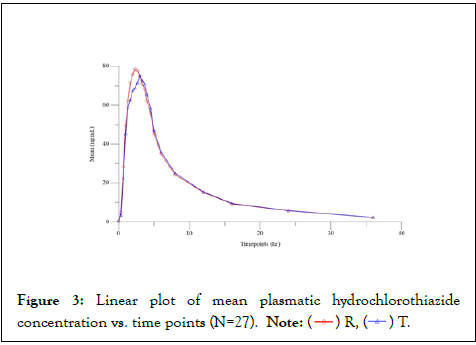
Figure 3: Linear plot of mean plasmatic hydrochlorothiazide concentration vs. time points (N=27). Note: ( ) R, (
) R, ( ) T.
) T.
Subjects drug administration and blood sampling
FAfter an overnight fasting of 10 hours, subjects were administered with a single oral dose of either test product or reference product with 200 mL of water according to the sequence assigned to each subject in sitting posture in each period. Compliance to drug administration was assessed by examination of the oral cavity and hands of the subject immediately after dosing. All the subjects remained in sitting posture for 02 hours after dosing. During this restriction period, the subjects were permitted to walk for natural exigencies. Subjects were restricted from consumption of water for 01 hour before and 01 hour after dosing in each period and allowed to drink water and libitum thereafter.
The pharmacokinetic profile of both test and reference products (in terms of rate and extent of absorption) was evaluated based on measured concentration of drug in the human plasma samples collected during the clinical phase. Blood samples for pharmacokinetic analysis were designed appropriately for characterizing the pharmacokinetic profile for the given treatments at the dose administered. A total of 21 Blood samples, of 04 mL each one, were collected at 00.00 (Pre-dose), 00.33, 00.67, 01.00, 01.33, 01.67, 02.00, 02.33, 02.67, 03.00, 03.33, 03.67, 04.00, 04.50, 05.00, 06.00, 08.00, 12.00, 16.00, 24.00 and 36.00 hours post dose for measurement of pharmacokinetic parameters. The samples collected were subjected to centrifugation and separated to 02 aliquots which were stored at -70°C. During bio analysis, the samples were thawed and a validated method was used to analyse the samples. Olmesartan was selectively extracted from human plasma by Solid Phase Extraction as per the in-house procedure; and Hydrochlorothiazide was selectively extracted from human plasma by Liquid-Liquid extraction as per the in-house procedure. The plasma samples were quantified using a validated bio-analytical method in LCMS/MS (Table 6).
| Parameter | N | Reference (R) (Mean ± SD) |
|---|---|---|
| Cmax (ng/mL) | 27 | 87.740 ± 19.644 |
| AUC0-t (hr.ng/mL) | 27 | 623.404 ± 139.395 |
| AUC0-∞ (hr.ng/mL) | 27 | 656.483 ± 148.213 |
| AUC%Extrap_Obs | 27 | 4.954 ± 1.372 |
| *Tmax (hr) | 27 | 2.33 (1.00-3.67) |
| T1/2 (hr) | 27 | 9.648 ± 1.136 |
| Kel (hr-1) | 27 | 0.073 ± 0.010 |
| VD (mL) | 27 | 280293.082 ± 78915.499 |
| CL (mL/hr) | 27 | 20201.085 ± 5513.725 |
Table 6: Summary of pharmacokinetic parameters for hydrochlorothiazide of reference product-R.
Results and Discussion
Tolerability
Subjects were monitored for adverse events during both periods of the study. Subjects were instructed to inform clinical personnel of any untoward medical symptoms and/or events that arose during the study. Each adverse event reported by the subjects during the study was assessed for its severity, relationship with the study drug and outcome of the adverse event. The Principal Investigator/subinvestigator/ physician also evaluated the subjects for subsequent dosing. Subjects’ Safety was assessed via continuous monitoring and scheduled recording of safety measurements throughout the study through clinical examinations, vital signs assessment, 12- lead Electrocardiogram (ECG), Rapid COVID-19 antigen test, clinical laboratory parameters (e.g., Haematology, Biochemistry, Urine analysis and Serology test) and monitoring subjects’ wellbeing, symptoms and signs for adverse events. All the subjects were assessed for their well-being throughout the conduct of the study. There were total of 02 non-serious adverse events reported by 02 subjects. Two adverse events were with test product and no adverse event was reported following administration of reference product. There were no serious adverse events reported during the study. All the adverse events resolved completely without any sequelae. The incidence of AEs in the study test product was (7.14%). The study medications were relatively well tolerated by the selected study population at the administered dose levels (Table 7).
| Parameter | N | Test (T) (Mean ± SD) |
|---|---|---|
| Cmax (ng/mL) | 27 | 87.674 ± 16.233 |
| AUC0-t (hr.ng/mL) | 27 | 619.917 ± 117.102 |
| AUC0-∞ (hr.ng/mL) | 27 | 652.819 ± 128.124 |
| AUC%Extrap_Obs | 27 | 4.879 ± 1.768 |
| *Tmax (hr) | 27 | 2.33 (1.00-4.00) |
| T1/2 (hr) | 27 | 9.325 ± 1.499 |
| Kel (hr-1) | 27 | 0.076 ±0.013 |
| VD (mL) | 27 | 264425.054 ± 53373.340 |
| CL (mL/hr) | 27 | 19918.769 ± 4177.356 |
Table 7: Summary of pharmacokinetic parameters for hydrochlorothiazide of test product-T.
Pharmacokinetic and statistical analysis
The pharmacokinetic and statistical analysis of Olmesartan and Hydrochlorothiazide were performed using the concentration data obtained from 27 subjects who completed both the periods of the study. In order to assess the two one-sided tests for bioequivalence, ratio analysis, 90% confidence intervals for the difference between treatments’ least-square mean was calculated for Ln-transformed Cmax, AUC0-t and AUC0-∞ of Olmesartan and Hydrochlorothiazide. Pharmacokinetic parameters were calculated using non-compartmental model of Phoneix® WinNolin® version 8.3 and statistical analysis was carried out using the SAS® statistical software, version 9.4 of SAS Institute Inc, USA. The mean, standard deviation, standard error, geometric mean, coefficient of variation, minimum, median, maximum and range were calculated for Cmax, AUC0-t, AUC0-∞, Tmax, t½, Kel, VD, CL and AUC_%Extrap_Obs Twenty-eight subjects in the age group of 20 to 44 years, who met the study eligibility criteria, participated in the study and twentyseven subjects completed the study. All the 28 subjects enrolled in the study were males and Asians. The recruitment of subjects to the study was open for both male and female subjects. However, as only male subjects are interested to participate in this study, only male subjects were included. This does not impact the study as the test product is comparable with that of the reference product and is interchangeable. One subject withdrew his consent due to personal reason in period II, hence was withdrawn. The clinical study was conducted over a period of 05 days. Blood sampling was done at pre-defined intervals up to 36.00 hours in both the periods, separated by a washout period of 02 days [8].
| Parameters | Cmax | AUC0-t | AUC0-∞ | Significance |
|---|---|---|---|---|
| Sequence effect | 0.968 | 0.7065 | 0.6767 | Insignificant for Cmax, AUC0-t and AUC0-∞ |
| Period effect | 0.3987 | 0.9975 | 0.9073 | Insignificant for Cmax ,AUC0-t and AUC0-∞ |
| Treatment (Formulation) effect | 0.9404 | 0.5454 | 0.667 | Insignificant for Cmax, AUC0-t and AUC0-∞ |
| Subjects nested within sequence effect | 0.0001 | <0.0001 | <0.0001 | Significant for Cmax, AUC0-t and AUC0-∞ |
Note: p<0.10 for Sequence effect and p<0.05 for all other effects considered to be significant
Table 8: p-value for Cmax and AUC of olmesartan.
| Parameters | Cmax | AUC0-t | AUC0-∞ | Significance |
|---|---|---|---|---|
| Sequence effect | 0.4221 | 0.4865 | 0.4977 | Insignificant for Cmax, AUC0-t and AUC0-∞ |
| Period effect | 0.3539 | 0.3973 | 0.4701 | Insignificant for Cmax , AUC0-t and AUC0-∞ |
| Treatment (Formulation) effect | 0.8364 | 0.9375 | 0.9507 | Insignificant for Cmax, AUC0-t and AUC0-∞ |
| Subjects nested within sequence effect | 0.0018 | <0.0001 | <0.0001 | Significant for Cmax, AUC0-t and AUC0-∞ |
Note: p<0.10 for Sequence effect and p<0.05 for all other effects considered to be significant
Table 9: p-value for Cmax and AUC of hydrochlorothiazide.
The pharmacokinetic plasma samples collected from 27 subjects were analysed to determine concentration of Olmesartan and Hydrochlorothiazide using a validated bio-analytical method in LCMS/MS. The pharmacokinetic and statistical analyses of Olmesartan and Hydrochlorothiazide were performed using the concentration data obtained from 27 subjects who completed both the periods of the study. Olmesartan is the 90% confidence interval of Cmax, AUC0-t and AUC0-∞ for Olmesartan was 92.31%- 107.60%, 91.81%-104.11% and 92.76%-104.56% respectively, which were within the acceptable limits of 80.00% to 125.00%. Hydrochlorothiazide: The 90% confidence interval of the relative mean Cmax, AUC0-t and AUC0-∞ of Hydrochlorothiazide were 94.40%-107.65%, 94.97%-105.83%, 95.00%-105.67% respectively, which were within the acceptable limits of 80.00 to 125.00%. The study was carried out in healthy male subjects. The tolerability of Olmesartan and Hydrochlorothiazide was already well established. As indicated in the package insert of the product, the study was conducted under fasting conditions. Olmesartan and Hydrochlorothiazide is expected to be less variable and the ISCV reported in the literature indicates the same. Hence, a conventional two-way crossover design was opted to evaluate the bioequivalence with optimal number of subjects. The washout period opted did not result in any carryover of the drug to subsequent period. Hence, the washout selected is considered adequate and accurate. The pharmacokinetic parameters estimated in the study are comparable with that of the published data. The sponsor, Abbott had included this study in the dossier submission for the generic drug approval in Chile (Figure 4) (Tables 8 and 9) [9].
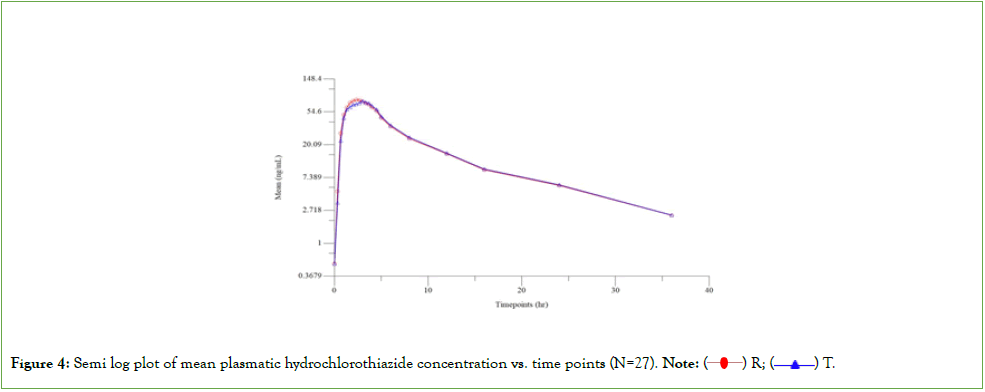
Figure 4: Semi log plot of mean plasmatic hydrochlorothiazide concentration vs. time points (N=27). Note: ( ) R, (
) R, ( ) T.
) T.
Conclusion
As a result, the washout chosen is deemed adequate and correct. The study's pharmacokinetic parameters are comparable to previously reported data. This study was included in the dossier application for generic medication approval in Chile by Abbott (sponsor). Bioequivalence was demonstrated between Olmesartan Medoxomil/Hydrochlorothiazide 20 mg/12.5 mg coated Tablets of Abbott and Olmetec Plus® (Olmesartan medoxomil/ Hydrochlorothiazide) 20 mg/12.5 mg coated Tablets of Daiichi- Sankyo, in healthy, adult, human subjects under fasting condition.
Acknowledgement
This scientific article was made with funding from Abbott, whose participation was related to financial support and document review. All technical, clinical and analytical execution of the bioequivalence studies were performed independently by Azidus Laboratories LTD.
References
- CDSCO’s New Drugs and Clinical Trials Rules. GSR. 2019.227(E).
- Harmonized Tripartite Guideline-Guideline for Good Clinical Practice (GCP).ICH.2016.
[Google Scholar] [PubMed]
- Structure and Content of Clinical Study Reports E3. Current Step 4 version. 1995.
- 21 Code of Federal Regulations. USFDA. 2023.
- Guide for performance of Comparative Bioavailability studies in solid pharmaceutical forms of oral administration and systemic action. ISP Chile - Technical guide G-BIOF 01.2018.
- Presentación de Resultados de Estudio de Biodisponibilidad/Bioequivalencia Para Estableer Equivalencia Terapéutica. Formulario F BIOF – 03.2011.
- Summary of product characteristics of Olmetec Plus® (Olmesartan Medoxomil/Hydrochlorothiazide) 20 mg/12.5 mg Coated Tablets of Daiichi- Sankyo.EMC.2022.
- Patient Information Leaflet of Olmetec Plus® (Olmesartan medoxomil/Hydrochlorothiazide) 20 mg/12.5 mg Coated Tablets of Daiichi- Sankyo. Olmetec Plus. 2022.
- Prescribing Information of BENICAR HCT® (Olmesartan medoxomil/Hydrochlorothiazide) 20 mg/12.5 mg Coated Tablets of Daiichi- Sankyo.Daily Med.2003.
Citation: Arumugam OA, Geetalakshmi G, Srinivas G, Karen H, Juliana HM, Arquímedes MG, et al. (2023) An Open Label, Balanced, Randomized, Two Treatments, Two Sequences, Two Periods, Crossover, Single Dose, Bioequivalence Study of Olmesartan medoxomil/Hydrochlorothiazide 20 mg/12.5 mg Coated Tablets of Abbott and Olmetec plus® (Olmesartan medoxomil/Hydrochlorothiazide) 20 mg/12.5 mg Coated Tablets of Daiichi-Sankyo in Healthy, Adult, Human Subjects Under Fasting Condition. J Bioequiv Availab. 15:538.
Copyright: ©2023 Arumugam OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited

