Indexed In
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Case Report - (2024) Volume 21, Issue 2
An HIV-Positive Woman Diagnosed with ALK Rearranged Lung Large-Cell Neuroendocrine Cancer with Unusual Metastasis
Hong Kang1, Fei Li2, Wangzhong Ye1, Shizhen Wu3 and Tian Yang4*2Department of Thyroid and Breast Surgery, Nanping First Hospital, Fujian, Nanping, China
3Department of Medicine Oncology, Nanping First Hospital, Fujian, Nanping, China
4Department of Radiation Oncology, Radiation Oncology Key Laboratory of Sichuan Province, Sichuan Clinical Research Center for Cancer, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Affiliated Cancer Hospital of University of Electronic Science and Technology of China, Chengdu, China
Received: 17-Jan-2024, Manuscript No. CMCH-24-24643; Editor assigned: 19-Jan-2024, Pre QC No. CMCH-24-24643 (PQ); Reviewed: 05-Feb-2024, QC No. CMCH-24-24643; Revised: 12-Feb-2024, Manuscript No. CMCH-24-24643 (R); Published: 19-Feb-2024, DOI: 10.35248/2090-7214.24.21.479
Abstract
Background: Pulmonary Large-Cell Neuroendocrine Carcinoma (LCNEC) is an exceedingly rare and aggressive type of lung cancer, often associated with metastasis to the brain, bones, adrenal glands and lymph nodes. To date, only a few cases of lung LCNEC with breast metastasis have been reported. Anaplastic Lymphoma Kinase (ALK) rearrangements occur in lung adenocarcinomas, but they are not typical for LCNEC and the median survival of LCNEC patients is approximately 1 year.
Case presentation: Here, we presented the case of an HIV-positive woman who was diagnosed with ALK-positive lung LCNEC from breast nodules. The patient responded dramatically to alectinib treatment. She showed an evidently longer median survival time than that reported previously, which suggests the benefit of using ALK inhibitors.
Conclusion: Based on our case experience, we recommend Next-Generation Sequencing (NGS) as a routine procedure for patients with LCNEC as it can provide more numbers of therapeutic alternatives.
Keywords
Breast metastasis; Pulmonary large cell neuroendocrine carcinoma; HIV; ALK rearrangement; Alectinib
Introduction
Pulmonary Large-Cell Neuroendocrine Carcinoma (LCNEC) is a rare type of lung cancer, accounting for <3.5% of all patients worldwide [1]. Breast metastasis of pulmonary LCNEC is extremely rare; therefore, most clinicians lack relevant experience in the diagnosis and treatment of this unique group of patients. According to past reports, breast metastases are rare and often derived from contralateral primary breast cancer.
Breast metastasis from extra mammary malignant tumors is unusual, with an incidence rate of 0.4%-1.3% among all breast metastases cases [2]. Anselmo et al, were the first to report a case of lung LCNEC that metastasized to the breast [3].
Currently, the treatment methods and prognosis of breast metastasis originating from extra mammary malignancies are different from those of primary breast cancer, which makes it even more critical to distinguish between these two subgroups.
Anaplastic Lymphoma Kinase (ALK) fusion has been reported in approximately 5%-6% of all Non-Small Cell Lung Cancer (NSCLC) patients, being more commonly observed in cases of lung adenocarcinoma [4]. However, reports on patients with ALK rearrangement in LCNEC are scarce. In fact, no study has yet reported lung LCNEC with ALK rearrangement and breast metastasis in an HIV-positive patient.
Here, we reported the case of a typical LCNEC patient who harbored an ALK rearrangement and displayed a dramatic response to alectinib. Subsequently, as the patient’s Performance Status (PS) continued to be satisfactory, she was deemed eligible for lorlatinib and further chemotherapy when alectinib treatment failed. This was the first case encountered at our cancer center. We have described here our unique observations to contribute toward the improvement of our understanding of breast metastasis, avoid potential misdiagnosis, and provide alternative or new treatment modalities for this disease.
Case Presentation
In July 2019, a 27-year-old female HIV-positive nonsmoker with no family history of breast cancer presented at our center with a hard mass in the left breast but no pulmonary symptoms. Her physical examination revealed symmetrical breasts with no nipple retraction or discharge, skin swelling, ulcers, or orange-peel appearance. There were no palpable masses in her right breast and double armpits, although there was a relatively firm lump measuring approximately 3.0 cm × 2.0 cm in the upper outer quadrant of her left breast. Ultrasound examination revealed a left breast nodule at 2 O’clock and 3 cm away from the nipple, measuring 30 mm × 10 mm × 19 mm. Enhanced Magnetic Resonance Imaging (MRI) of her breasts revealed a left-breast mass patchy enhancement lesion of dimensions 24 mm × 13 mm × 18 mm (Figure 1).
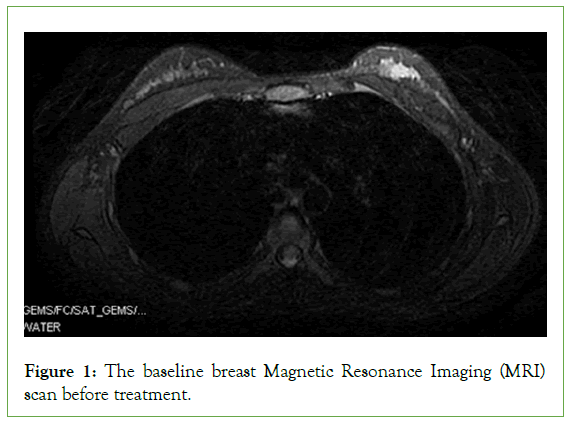
Figure 1: The baseline breast Magnetic Resonance Imaging (MRI) scan before treatment.
The left breast tumor was removed via ultrasound-guided minimally invasive atherectomy and dispatched for pathological examination. Immunohistochemistry (IHC) results revealed that E-C and P120 were positive and ER, PR, CK5/6, P63, and HER-2 were negative, while the Ki67 proliferation index was 40%. Subsequently, the enhanced chest Computed Tomography (CT) scan (Figure 2), revealed a 31 mm × 26 mm tumor in the lower lobe of the left lung (Figure 2A). The pathological lung diagnosis was a poorly differentiated neuroendocrine tumor with positive CgA, Syn, and CD56 expression consistent with LCNEC. Concurrently, the left breast pathology along with IHC results indicated the positive expression of CgA, Syn, CD56, TTF-1, and ALK-V, while the clinical stage was established as stage IV. Considering the age and immunodeficient condition of the patient, NGS was conducted to aid in appropriate treatment-related decision-making. NGS of the left lung tumor tissue and blood specimens demonstrated an ALK-EML4 fusion with mutation frequencies of 46.2% and 0.15%, respectively.
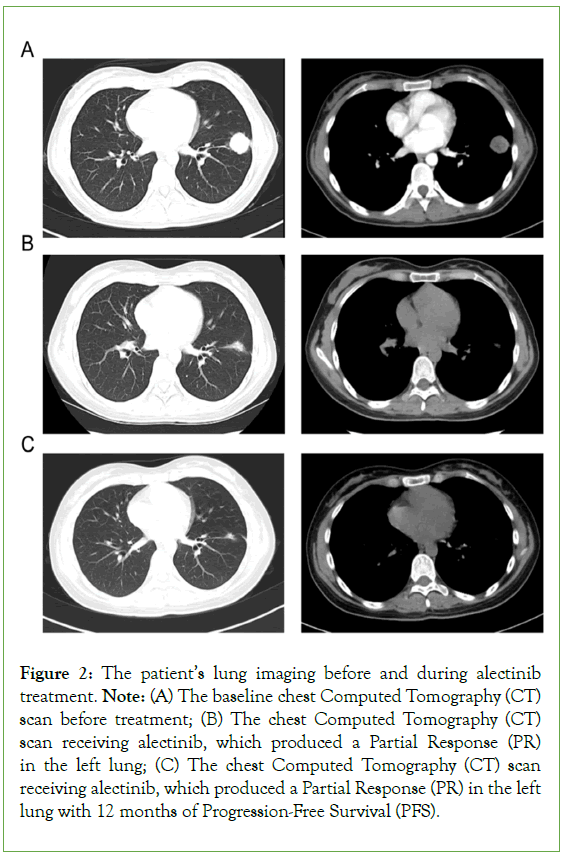
Figure 2: The patient’s lung imaging before and during alectinib treatment. Note: (A) The baseline chest Computed Tomography (CT) scan before treatment; (B) The chest Computed Tomography (CT) scan receiving alectinib, which produced a Partial Response (PR) in the left lung; (C) The chest Computed Tomography (CT) scan receiving alectinib, which produced a Partial Response (PR) in the left lung with 12 months of Progression-Free Survival (PFS).
The first-line regimen alectinib which is a novel and highly selective inhibitor of ALK translocation was given to the patient at first. After 4 months of alectinib treatment, CT imaging confirmed the achievement of a Partial Response (PR) (Figure 2B). Her physical examination revealed no palpable masses in the bilateral breasts. After a year, her chest CT scan confirmed the stale state of her left lung lesion (Figure 2C). However, MRI revealed brain metastasis (Figure 3A). Accordingly, the patient received brain gamma knife treatment. Subsequently, the enhanced chest CT scan revealed a 21 mm × 29 mm tumor in the hilum of her left lung (Figure 3B). The breast ultrasound revealed a hypoechoic mass measuring 30 mm × 10 mm × 19 mm in the right breast (Figure 3C), displaying similar histology of tumor cells to the original lung biopsy (Figure 3). Considering the resistance to alectinib, lorlatinib was administered in the second-line regimen, which is a potent, brain- penetrant, third-generation inhibitor of ALK and ROS1 tyrosine kinases, that has become available for ALK-positive advanced NSCLC patients who relapse or show resistance to ALK inhibitors. After 5 months of lorlatinib treatment, the radiological evaluation revealed continued shrinkage of the brain metastasis (Figure 4A), and lung lesion (Figure 4B); thus, the patient was deemed to have achieved a PR. Unfortunately, the patient reported experiencing pain in her right breast. Her physical examination revealed skin swelling and thickening of the entire right breast. In addition, the breast ultrasound scan revealed a new progression in the right breast (Figure 4C). The patient refused to undergo surgery owing to her immunodeficient state. Consequently, the right breast tumor received stereotactic body radiotherapy at 40 Gy in 5 fractions, 8 Gy/fraction, to the right breast with 6-MV photons. After 9 months of treatment with lorlatinib, routine ultrasound confirmed that the left breast lesion was stable, but the breast ultrasound scan indicated progression in the right breast (Figure 4D). Therefore, two cycles of chemotherapy with cisplatin and etoposide were administered. Presently, the patient’s PS is good, except for swelling and pain in the right breast.
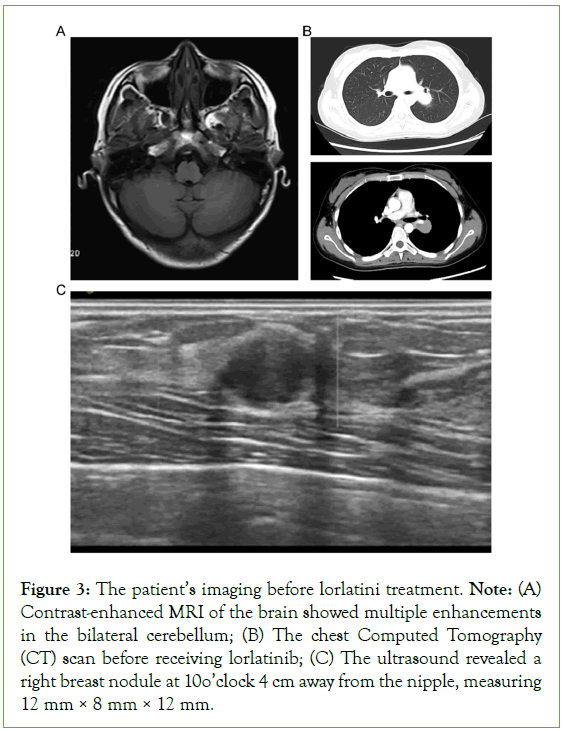
Figure 3: The patient’s imaging before lorlatini treatment. Note: (A) Contrast-enhanced MRI of the brain showed multiple enhancements in the bilateral cerebellum; (B) The chest Computed Tomography (CT) scan before receiving lorlatinib; (C) The ultrasound revealed a right breast nodule at 10 o’clock 4 cm away from the nipple, measuring 12 mm × 8 mm × 12 mm.
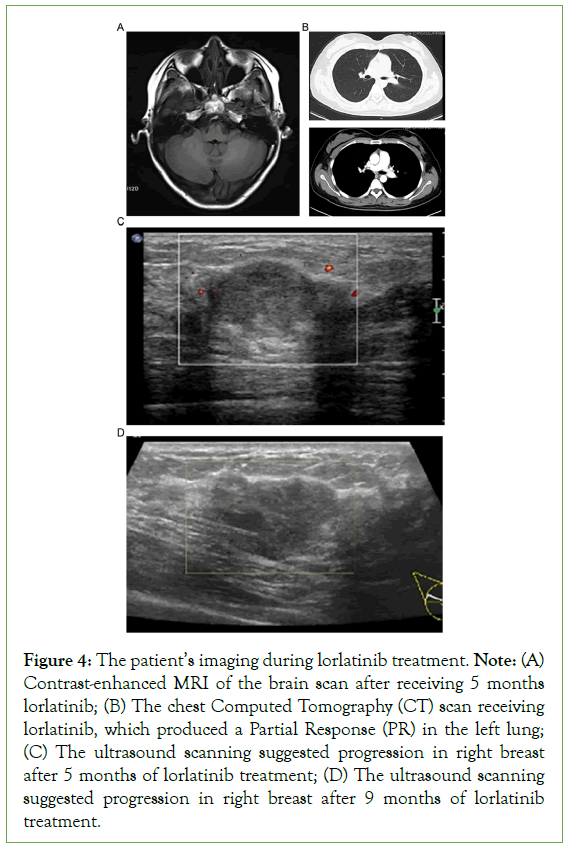
Figure 4: The patient’s imaging during lorlatinib treatment. Note: (A) Contrast-enhanced MRI of the brain scan after receiving 5 months lorlatinib; (B) The chest Computed Tomography (CT) scan receiving lorlatinib, which produced a Partial Response (PR) in the left lung; (C) The ultrasound scanning suggested progression in right breast after 5 months of lorlatinib treatment; (D) The ultrasound scanning suggested progression in right breast after 9 months of lorlatinib treatment.
After 3 years of treatment, presently, the patient has only grade 1 adverse reaction in accordance with the evaluation criteria for common adverse events of drugs Common Terminology Criteria for Adverse Events (CTCAE 5.0). Her clinical conditions have improved, her left breast nodule has disappeared, and she is delighted with the current treatment.
Results and Discussion
Breast cancer is one of the most frequent primary malignancies affecting women, but breast metastases from extra mammary malignancies are extremely rare and often associated with poor prognosis [5]. Stephanie et al, reported that the median survival duration of metastatic breast cancer patients was only 10 months [6]. The present case patient was diagnosed with a breast nodule, which could have been easily misdiagnosed as primary breast cancer. Fortunately, the patient received an accurate diagnosis and was recommended an optimal treatment after a definite pathological diagnosis. Consequently, a pathological examination is necessary to differentiate primary breast cancer from metastatic tumors. Our patient survived 3 years following the diagnosis of lung LCNEC with breast metastasis, which is inconsistent with any past report. Therefore, clinicians should consider the possibility of breast metastases when encountering patients with no previous history of breast cancer or other malignancies.
Since lung LCNEC is a rare type of lung cancer, the related reports are scarce and mainly derived from small sample studies. Moreover, a comprehensive analysis was limited, and the clinical management for LCNEC remains controversial [7,8]. Driving genes such as Epidermal Growth Factor Receptor (EGFR) and ALK-forecasted targeted therapy in NSCLC are both rare. Shen et al, reported the mutation rates of EGFR and ALK as 8.33% and 5.77%, respectively [9]. In addition, only a few studies have reported ALK rearrangement in lung LCNEC [10,11], and only little is known regarding the clinical features of these patients. Although most patients with advanced LCNEC generally receive platinum-based doublet chemotherapy as the first-line regimen, the therapeutic results are unsatisfactory and the prognosis is poor [12,13]. Anselmo et al, described the first LCNEC case with breast metastasis in 2015; unfortunately, NGS was not performed on their patient as it was not typically recommended for this subtype, and their 59-year-old female patient died within 5 months of receiving chemotherapy [3]. Our ALK-positive patient received alectinib and achieved a PR for 12 months, but the metastatic brain tumor acquired resistance to ALK-TKIs, as also reported previously in a case [14]. Recently, occasional ALK rearrangements in LCNEC cases with dramatic reactions to ALK‐TKIs have been reported [15,16]. In the present case, the tumor in the right breast progressed, but that in the lung and brain remained stable after receiving the second ALK-Is lorlatinib.
The increased risk of lung cancer in HIV-infected persons is possibly related to the following mechanisms: HIV-induced immunodeficiency and HIV-associated decrease in immune surveillance. Takahiro et al, reported the achievement of PR in their patient with primary and metastatic lesions through alectinib therapy; however, the tumor in brain metastases progressed after 11 months of this treatment [14]. The present study provides the first evidence of the coexistence of a distinct evolution of Tumor-Immune Microenvironments (TIMEs) within an ALK rearrangement-positive LCNEC patient. Based on the cumulative observations and findings, we considered that the right breast tumor progression in our patient was associated with TIMEs influenced by the HIV-positive feature, which could have resulted in immunodeficiency.
Conclusion
Breast metastasis from extra mammary malignancy is rare, and it is critical to establish appropriate treatment and prediction of its prognosis. Pathological examination is vital for distinguishing primary breast cancer from metastatic tumors. Based on our case experience, although ALK rearrangement is a rare phenomenon in LCNEC, further driver mutation tests of patients with lung LCNEC may provide helpful insights into the diagnosis for designing optimal treatment strategies against the tumor.
Declarations
Ethical approval and consent
The patient consented to provide treatments as per institutional and national guidelines. Written informed consent was obtained from the individual participant included in the study and the patient consented to submission and publication of this case report.
Competing interests
The authors declare no conflict of interest.
Authors’ contributions
Study conception and design: HK, FL and TY. Acquisition of data: HK and HK. Analysis and interpretation of data: WZY and SZW. Drafting of manuscript: HK, FL and TY. Critical revision of manuscript: HK, FL and TY. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the set sail funding of Fujian Medical University (grant number: 2021QH1222)
Availability of data and materials
The data are available from the corresponding author, T.Y, upon reasonable request.
References
- Fasano M, Corte CMD, Papaccio F, Ciardiello F, Morgillo F. Pulmonary large-cell neuroendocrine carcinoma: From epidemiology to therapy. J Thorac Oncol. 2015;10(8):1133-1141.
[Crossref] [Google Scholar] [PubMed]
- Martin-Sanchez JC, Lunet N, Gonzalez-Marron A, Lidon-Moyano C, Matilla-Santander N, Cleries R, et al. Projections in breast and lung cancer mortality among women: A Bayesian analysis of 52 countries worldwide. Cancer Res. 2018;78:4436-4442.
[Crossref] [Google Scholar] [PubMed]
- Papa A, Rossi L, Verrico M, Di Cristofano C, Moretti V, Strudel M, et al. Breast metastasis and lung large-cell neuroendocrine carcinoma: first clinical observation. Clin Respir J. 2015;09;00:000-000.
[Crossref] [Google Scholar] [PubMed]
- Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-Rearrangement in Non-Small-Cell Lung Cancer (NSCLC). Thorac Cancer. 2018;9(4):423-430.
[Crossref] [Google Scholar] [PubMed]
- Bitencourt AGV, Gama RRM, Graziano L, Negrao EMS, Sabino SMPS, Watanabe AHU, et al. Breast metastases from extra mammary malignancies: Multimodality imaging aspects. Br J Radiol. 2017;90.
[Crossref] [Google Scholar] [PubMed]
- Williams SA, Ehlers 2nd RA, Hunt KK, Yi M, Kuerer HM, Singletary SE, et al. Metastases to the breast from nonbreast solid neoplasms: Presentation and determinants of survival. Cancer. 2007;110:731-737.
[Crossref] [Google Scholar] [PubMed]
- Zhang JT, Li Y, Yan LX, Zhu ZF, Dong XR, Chu Q, et al. Disparity in clinical outcomes between pure and combined pulmonary large-cell neuroendocrine carcinoma: A multi-center retrospective study. Lung Cancer. 2020;139:118-123.
[Crossref] [Google Scholar] [PubMed]
- Zhuo M, Guan Y, Yang X, Hong L, Wang Y, Li Z, et al. The prognostic and therapeutic role of genomic subtyping by sequencing tumor or cell-free DNA in pulmonary large-cell neuroendocrine carcinoma. Clin Cancer Res. 2020;26:892-901.
[Crossref] [Google Scholar] [PubMed]
- Shen Y, Hu F, Li C, Xu J, Zhong R, Zhang X, et al. Clinical features and outcomes analysis of surgical resected pulmonary large-cell neuroendocrine carcinoma with adjuvant chemotherapy. Front oncol. 2020;10:556194.
[Crossref] [Google Scholar] [PubMed]
- Hayashi N, Fujita A, Saikai T, Takabatake H, Sotoshiro M, Sekine K, et al. Large cell neuroendocrine carcinoma harboring an Anaplastic Lymphoma Kinase (ALK) rearrangement with response to alectinib. Intern Med. 2018;57(5):713-716.
[Crossref] [Google Scholar] [PubMed]
- Shimizu N, Akashi Y, Fujii T, Shiono H, Yane K, Kitahara T, et al. Use of ALK immunohistochemistry for optimal therapeutic strategy of pulmonary large-cell neuroendocrine carcinoma and identification of a novel KIF5B–ALK fusion oncokinase. Anticancer Res.2019;39(1):413-420.
[Crossref] [Google Scholar] [PubMed]
- He Y, Liu H, Wang S, Chen Y. Prognostic nomogram predicts overall survival in pulmonary large cell neuroendocrine carcinoma. PLoS ONE. 2019;14:e0223275.
[Crossref] [Google Scholar] [PubMed]
- Klein O, Kee D, Markman B, Michael M, Underhill C, Carlino MS, et al. Immunotherapy of Ipilimumab and Nivolumab in patients with advanced neuroendocrine tumors: A subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res. 2020;26:4454-4459.
[Crossref] [Google Scholar] [PubMed]
- Tashiro T, Imamura K, Tomita Y, Tamanoi D, Takaki A, Sugahara K, et al. Heterogeneous tumor-immune microenvironments between primary and metastatic tumors in a patient with ALK rearrangement-positive large cell neuroendocrine carcinoma. Int J Mol Sci. 2020;21(24):9705.
[Crossref] [Google Scholar] [PubMed]
- Zaixiang Fu, Ganggui Zhu, Liquan Wang, Shen Hu, Lu Cheng, Fuyi Liu. Case report: A pregnant woman diagnosed as ALK-rearrangement lung large cell neuroendocrine cancer with brain metastasis. Front Oncol. 2022;12:823813.
[Crossref] [Google Scholar] [PubMed]
- Mengoli MC, Bertolini F, Maur M, Barbieri F, Longo L, Gasparri P, et al. ALK-positive adenocarcinoma of the lung expressing neuroendocrine markers and presenting as a “Pituitary Adenoma”. Pathologica. 2017;109(4):408-411.
[Crossref] [Google Scholar] [PubMed]
Citation: Kang H, Li F, Ye W, Wu S, Yang T (2024) An HIV-Positive Woman Diagnosed with ALK Rearranged Lung Large-Cell Neuroendocrine Cancer with Unusual Metastasis. Clinics Mother Child Health. 21:479.
Copyright: © 2024 Yang T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

