Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- Scientific Indexing Services (SIS)
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Commentary - (2021) Volume 12, Issue 6
Advances in Viable Ice-free Cryopreservation of Heart Valves
Kelvin G.M. Brockbank*2Department of Bioengineering, Clemson University, Charleston, SC, USA
3Department of Regenerative Medicine and Cell Biology, Medical University of South Carolina, SC, USA
Received: 15-May-2021 Published: 28-Apr-2021, DOI: 10.35248/2157-7439.21.12.566
Abstract
Investigation of heart valve cryopreservation has been employed as a model for development of new methods of tissue preservation based upon vitrification and nanowarming using Fe nanoparticles. Cryoprotectant cytotoxicity can be reduced by performing the last cryoprotectant/nanoparticle exposure step below zero degrees centigrade at -10C. Tissue viability outcomes can be improved by supplementation of cryoprotectant formulations with disaccharides and nanowarming can rewarm such complex tissues with retention of cell viability from storage temperatures below -135ºC to -25ºC in 80-100 seconds. It is anticipated that ice-free tissue cryopreservation methods for tissues up to 50 mLs can be developed that do not require the use of nanowarming, since we are already close to achieving this with heart valves at 30 mL volumes. However, at larger volumes nanowarming will likely continue to be the best warming method for retention of tissue cell viability. Further studies to optimize cryopreservation of cardiac muscle, the somewhat fibrous muscle band at the base of heart valves, and pulmonary and aortic arteries need to be performed since it is clear that different heart valve components vary in their preservation requirements. It is anticipated that other complex tissues may also have components with different cryopreservation requirements including nanowarming.
Keywords
Cryopreservation; Nanowarming; Nanoparticles; Cytotoxicity
Introduction
We have developed methods for ice-free cryopreservation of tissues that retain heart valve extracellular matrix and leaflet cell viability is an option depending upon the cryoprotectant formulation employed [1]. The non-viable methods result in minimization of recipient immune reaction and good allograft function in large animals. However, there is a huge unfulfilled military and civilian need for viable banked natural and bioengineered tissues and organs for replacement of disease or trauma-induced damage to body parts [2-3]. Investigation of heart valve cryopreservation methods with retention of cell viability in the leaflets, associated artery and fibrous muscle band has been employed as a model for development of new methods of tissue preservation based upon vitrification.
Vitrification, cryopreserved storage in a “glassy” rather than crystalline phase, is an important enabling approach for tissue banking and regenerative medicine, offering the ability to store and transport body parts for a variety of biomedical uses. Unfortunately, practical application of vitrification has been limited to cells and thin tissues in small volumes of cryoprotectant solutions due to diffusive (heat and mass transfer) and phase change limitations that preclude use in bulk systems such as organs and larger tissues. Greg Fahy developed a vitrification cryoprotectant formulation for tissues and organs in the 1980’s consisting of 55% cryoprotectants in Euro Collins organ preservation solution [4]. In the year 2000, our successful VS55 ice-free cryopreservation of rabbit blood vessels with retention of cell viability and tissue contractile functions was published [5] but in the ensuing years we could not scale up to volumes > 3-4mLs. In the past 5 years two breakthrough strategies were developed to circumvent this problem. First, the use of radiofrequency (RF) excited magnetic nanoparticles (mNPs) in cryoprotectant solution to aid in rewarming of tissues developed by the Bischof laboratory at the University of Minnesota. This approach relies on uniform heat generation in close proximity to or within the biomaterial and overcomes the fundamental limitations experienced with boundary or microwave heating in the past. The most important opportunity for this new warming technology is rapid rewarming after ice-free cryopreservation of large tissues and in the future organs. Organ cryopreservation is currently limited by cracking during storage or rewarming and ice formation due to devitrification during warming in a liquid bath. Furthermore, faster rewarming rates may reduce the risks of exposure to the high concentrations of cytotoxic cryoprotective agents needed to avoid devitrification. The second strategy, developed by the Brockbank laboratory at Tissue Testing Technologies, was to make the lead ice-free vitrification solution, VS55, less likely to devitrify during rewarming by supplementation with disaccharide sugars.
Our studies with RF coated Fe mNPs in collaboration with the Bischof laboratory at the University of Minnesota were published in 2017 [6]. However in the same time frame we discovered the benefits of two disaccharides in combination with VS49, DP6 and VS55 vitrification formulations (Brockbank patent pending, Figure.1, see Table I for formulations). The impact of sucrose on prevention of devitrification was demonstrated by differential scanning calorimetry [7]. At that time VS55 was our best ice-free vitrification formulation, while a dilute version of this formulation, VS49, that does not vitrify was used as a negative control. Similarly, DP6 is also a poor solution for vitrification in which formamide has been removed and equimolar concentrations of DMSO and propanediol are employed to produce a 6M cryoprotectant formulation. The viability outcomes are poor for the leaflet and conduit components of heart valves preserved in these negative control solutions (Figure 1, top panel). Addition of 0.6M disaccharides prevents visible ice formation in both DP6 and VS49 formulations and we observed some of the highest post-vitrification viability results to date in DP6, VS49 and VS55 plus 0.6M disaccharides (Figure 1). The presence of 0.6M trehalose and particularly 0.6M sucrose increased post-rewarming leaflet viability 2 to 3-fold achieving statistical significance, compared with the best outcomes we could achieve employing VS55 without disaccharides (Figure 1, middle and lower panels, p<0.05 by T-test). A significant increase was also observed for pulmonary artery conduit components of the heart valves (Figure 1). Sucrose combined with VS55 yielded the highest post-cryopreservation viabilities.
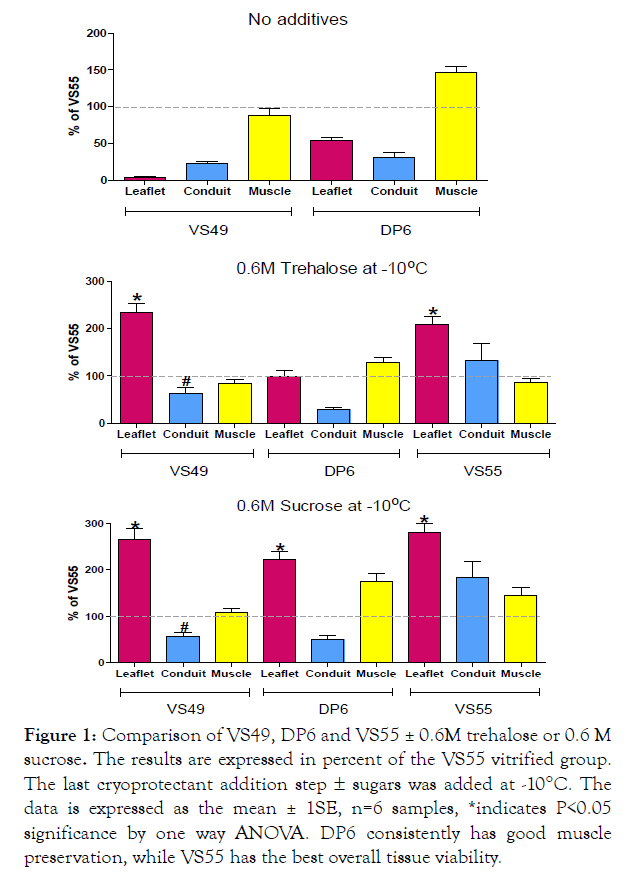
Figure 1: Comparison of VS49, DP6 and VS55 ± 0.6M trehalose or 0.6 M sucrose. The results are expressed in percent of the VS55 vitrified group. The last cryoprotectant addition step ± sugars was added at - 10°C. The data is expressed as the mean ± 1SE, n=6 samples, *indicates P<0.05 significance by one way ANOVA. DP6 consistently has good muscle preservation, while VS55 has the best overall tissue viability.
| Components | DP6 | VS49 | VS55 |
|---|---|---|---|
| Dimethylsulfoxide (DMSO) (M) | 3.0 | 2.7 | 3.1 |
| Propanediol (M) | 3.0 | 1.9 | 2.2 |
| Formamide (M) | 2.7 | 3.1 |
Table 1: Vitrification Formulations(in Euro Collins Solution).
We published our follow up experiments in which we combined our sucrose VS55 cryopreservation strategy with nanowarming in a review of ice-free heart valve cryopreservation in 2021 [8]. The combination of 0.3 M sucrose and 0.3 M trehalose produced similar results comparing convection with nanowarming (using Ferrotec EMG308 at 7.69 mg Fe/mL) for leaflets but the conduit and muscle band viabilities were significantly higher post-nanowarming (Figure 2). The thicker less cryoprotectant permeable tissue components, conduit and muscle band, are probably less permeated by the cryoprotectants than the leaflets. Therefore, these tissues benefit from the rapid warming possible using nanowarming preventing ice formation due to devitrification during warming. Work in progress is focused on improved cryoprotectant loading strategies for large arteries and the muscle band at the base of pulmonary heart valves.
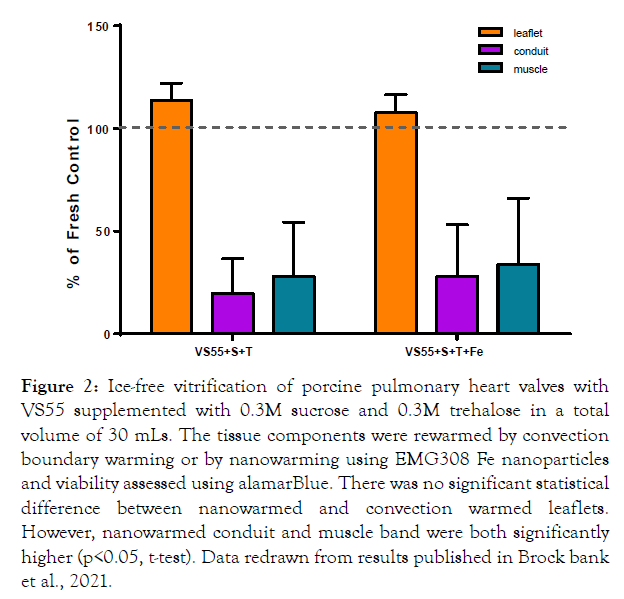
Figure 2: Ice-free vitrification of porcine pulmonary heart valves with VS55 supplemented with 0.3M sucrose and 0.3M trehalose in a total volume of 30 mLs. The tissue components were rewarmed by convection boundary warming or by nanowarming using EMG308 Fe nanoparticles and viability assessed using alamarBlue. There was no significant statistical difference between nanowarmed and convection warmed leaflets. However, nanowarmed conduit and muscle band were both significantly higher (p<0.05, t-test). Data redrawn from results published in Brock bank et al., 2021.
In conclusion results of studies to improve cell viability in pulmonary heart valves demonstrate that:
• Cryoprotectant cytotoxicity can be reduced by performing the last cryoprotectant/nanoparticle exposure step below zero degrees centigrade at -10C (Figure 1). Colder temperatures can also be used.
• Tissue viability outcomes can be improved by supplementation of cryoprotectant formulations with disaccharides (Figure 1).
• Nanowarming can rewarm such complex tissues with retention of cell viability from nitrogen storage temperatures below -135°C to -25°C in 80-100 seconds (Figure 2).
It is anticipated that ice-free tissue cryopreservation methods for tissues up to 50 mLs in volume, tissue and surrounding solution combined, can be developed that do not require the use of nanowarming, since we are already close to achieving this with heart valves at 30 mL volumes (Figure 2). However, at larger volumes nanowarming will likely continue to be the best warming method for retention of tissue cell viability. Further studies to optimize cryopreservation of cardiac muscle, the somewhat fibrous muscle band at the base of heart valves, and pulmonary and aortic arteries needs to be performed since it is clear that different heart valve components vary in their preservation requirements. It is anticipated that other complex tissues may also have components with different cryopreservation requirements. Biomaterial testing and in vivo large animal tissue transplant studies employing ice-free cryopreservation methods and nanowarming with retention of cell viability are in progress.
Acknowledgements
This work was funded by the U.S. Army Medical Research and Materiel Command (contract no. W81XWH-15-C-0173 and W81XWH- 16-C-0074). The views, opinions, and findings contained in this report are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation. The commercial uses of protocols disclosed in this work are subject to several issued US Patents (6,194,137; 6,596,531; 6,740,484; 7,157,222; 8,440,390), International Patents (available upon request), and pending unpublished patents.
REFERENCES
- Brockbank KGM, Chen Z, Greene ED, Campbell LH. Vitrification of heart valve tissues. In: Methods in Cryopreservation and Freeze-Drying, Editor(s): Wolkers WF, Oldenhof H. Methods Mol Biol. 2015.
- Lewis, Bischof, Braslavky, Brockbank. The Grand Challenges of Organ Banking: Proceedings from the first global summit on complex tissue cryopreservation, Cryobiology, 2015.
- Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Brandacher, et al. The promise of organ and tissue preservation to transform medicine. Nat Biotechnol 2017;35 (7):530–542.
- Fahy GM. Verification as an approach to organ cryopreservation: Past, present, and future, in Cryopreservation and Low Temperature Biology in Blood Transfusion, Smit Sibinga C.T, Das P.C, and Meryman H.T, Eds Kluwer Academic Publishers, Dordrecht, pp. 255–268, 19889.
- Song YC, Khirabadi BS, Lightfoot F, Brockbank KGM, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nat Biotechnol. 2000;18:3: 296- 299.
- Manuchehrabadi N, Gao Z, Zhang J, Ring HL, Shao Q, Liu F, et al. Improved Tissue Cryopreservation using Nanowarming: Inductive Heating of Magnetic Nanoparticles. Sci Transl Med. 2017;9(379).
- Phatak S, Natesan H, Choi J, Brockbank KGM, Bischof JC. Measurement of Specific Heat and Crystallization in VS55, DP6 and M22 Cryoprotectant Systems with and without Sucrose. Biopreserv Biobank. 2018;16(4):270-277.
- Brockbank KGM, Chen Z, Greene ED, Campbell LH. Verification of heart valve tissues. In: Cryopreservation and Freeze-‐Drying Protocols, Fourth Edition, Editor(s): Wolkers WF, Oldenh of H, Methods Mol Biol. Springer. 2021;31:593-605.
Citation: Brockbank KGM, Chen Z, Greene ED, Campbell LH (2021) Advances in Viable Ice-free Cryopreservation of Heart Valves. J Nanomed Nanotech. 12: 566.
Copyright: ©2021 Brockbank KGM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work was funded by the U.S. Army Medical Research and Materiel Command (contract no. W81XWH-15-C-0173 and W81XWH-16-C-0074). The views, opinions, and findings contained in this report are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation. The commercial uses of protocols disclosed in this work are subject to several issued US Patents (6,194,137; 6,596,531; 6,740,484; 7,157,222; 8,440,390), International Patents (available upon request), and pending unpublished patents.


