Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2020) Volume 11, Issue 6
A Study on the Reaction of Some Grapevine Varieties to Crown Gall Agent (Agrobacterium vitis)
Fadaei AA* and Fathi SHReceived: 27-Mar-2020 Published: 10-Jun-2020, DOI: 10.35248/2157-7471.20.11.498
Abstract
Grapevine is one of the oldest and most economically fruit crops. Grapes are a rich source of vitamins A, C, B6, as well as essential minerals, such as potassium, calcium, iron, phosphorus, magnesium and selenium. Crown gall disease (Agrobacterium vitis) is an economical disease in most vine yards. Since the bacterium remains in soil for a long time control of Agrobacterium is very difficult. Use of resistant rootstocks is the most effective methods for control of soil-borne pathogens, especially this bacterium. In this study, the reaction of the eight grape varieties (Shahani, Askari, Rish baba, Sefid-e-yaghuti, Qazvin Sefid-e-Keshmeshi, Qazvin Ghermez-e-Keshmeshi, Mehre and Rotabi) to crown gall was studied. In the first experiment, the rooted cuttings of different varieties inoculated in four sections with 20 ml of 108 cfu A. vitis and distillated water were compared as factorial experiment in a completely randomized design with four replications in green house. In the second set, inoculations were made by adding 40 ml of the suspension of two strains of bacteria (with the same concentration) around the root. Evaluations were made by growth and pathogenicity indices after five months. The callus formation on shoots was also studied in MS medium with and without bacterium. The results indicated that no varieties were immune to crown gall. Analysis of variance and mean comparisons of growth, physiological and pathogenicity indices showed the significant reduction in dry and wet weight of shoots and photosynthetic pigments in Shahni, sefid-e-yaquti and Rotabi. The soluble carbohydrate and anthocyanin also increased in these varieties. The highest necrosis, callus and gall formation were observed in Sefid-e-Yaghuti. It can be concluded that the Shahani, Rotabi and Sefid-e-Yaghuti varieties are most susceptible to causal agent of crown gall.
Keywords
Agrobacterium vitis; Crown gall
Introduction
Crown gall is a serious disease of grapevines worldwide and is particularly severe in region where vineyards undergo freeze injury [1]. When crown gall is intense, vine vigor and productivity e significantly decrease [2] leading to considerable mortality of young vines in cold winter regions [3]. The strains of Agrobacterium associated with most grape crown galls are separate from other crown gall pathogens and had been grouped together as Agrobacterium tumefaciens (AT), biovar 3 [4]. They are now distinguished as Agrobacterium vitis (AV), separated from other Agrobacterium species by differences in DNA homology and metabolic characteristics [5]. Since crown gall-susceptible vines seemingly allow A. vitis entry through roots [6], the use of resistant scion may decrease infection from soil inoculum and forbid subsequent infection of A. vitis free but highly crown gallsusceptible rootstocks cultivars.
Resistance to crown gall in vitis was reported by many researchers. Szegedi et al. [7] reported that elections of three Asian vitis species, V. amurensis, V. piasezkii, and V. flexuosa, formed no galls when inoculated with strains of A. vitis from Hungary (Like these strains were tumorigenic on V. vinifera. Crown gall resistance of V. amurens is reported to be dominant in crosses with V. vinifera [7]. Stover et al. [8] inoculated 40 various scions with five A. vitis strains and found that P775 was immune while small galls formed on 3309C and 101-14 Mgt, among others, following inoculation with some of the strains studied. Utilization of resistant rootstocks may reduce the incidence of crown gall when A. vitis free vines are planted on A. vitis polluted site. The purpose of this study was to determine the response of grape varieties of crown gall and to identify the rootstocks which are resistant to crown gall.
Materials and Methods
Plant material
Eight grape varieties (i.e., Shahani, Askari, Rish baba, Sefid-eyaghuti, Qazvin Sefid-e-Keshmeshi, Qazvin Ghermez-e- Keshmeshi, Mehre and Rotabi) were obtained from the Isfahan University of Technology’s farm. Rootstocks were held in sandy sweet in the greenhouse until they were rooted. Young plants were inoculated with A. vitis after the appearance of brown periderm. Typically shoots were 30-40cm long at the time of inoculation. The sites of inoculation were in the green space node.
Agrobacterium strain
Agrobacterium vitis strains were kept at -80°C in cryogenic storage medium.
Isolation of A. vitis
Vineyards and nurseries of grapevine (vitis spp.) were inspected for crown gall occurrence in Chaharmahal va bakhtiyari in Iran at the spring and autumn seasons. Samples of newly developed galls from the infected plants were collected and transported to the laboratory. Plant samples were washed with running tap water to remove soil particles, surface sterilized by dipping into 0.5% v:v sodium hypochlorite for 2 min, and rinsed three times with sterile distilled water (SDW).
Small portions were aseptically removed from each sample and placed in few drops of SDW in a mortar and pestle for maceration. The resulting suspension was left to stand for 30 min, and then loop full of the gall suspensions were streaked on RS medium [9]. Inoculated plates were incubated at 28°C until bacterial growth developed. Colonies consistent with A. vitis after 5 days on RS medium were streaked on potato dextrose agar (PDA).
Identification of A. vitis
Tumorigenicity: The ability of the bacterial isolates to induce galls was tested on stems of tomato (Lycopersicon esculentum). Inoculum was prepared by suspending a loop full of bacterial growth from a 28-hold PDA slant culture into 2 ml distilled water. Plants grown in a greenhouse were inoculated at 2-4 sites per internode by pricking through a drop of inoculum with a needle.
The pathogenicity was determined 30 days after inoculation based on gall formation at the site of pricking. Also ability of this bacterium to callus formation was determined by carrot (Daucus carota) disks. Fresh carrot root disks were sterilized and inoculated by 30-40 ml bacterial suspension 108cfu on a moist filter paper in a petri plate [10,11].
Phenotypical characterization tests: Some phenotypical and biochemical tests for the identification and differentiation of A. vitis from other species were made as mentioned below.
Callus formation: Young actively-growing shoots were removed from plants and surface sterilized in 0.5% sodium hypochlorite for 10 min and then rinsed three times in SDW. Exposed ends of explants were removed. Nodal explants were placed in MS (Murashige & Skoog) medium in culture jars without regulator growth. 5 μL of the A. vitis 339-26 suspension (108 cfu/ml) were spread over the exposed cut surface of each explant. As a control internodes were placed on MS medium with 0.049 μM indole butyric acid (IBA). Culture jars were kept in dim light and explants were sampled after 2 weeks inoculation.
Ability to induction grape necrosis: The ability of isolates to cause necrosis was determined on grape shoot explants. Actively growing shoots from potted vines in the greenhouse were harvested, and then surface disinfected by submersion in 0.5% sodium hypochlorite for 10 min and then rinsing with sterile distilled water.
Internodal sections were cut and supported vertically in 1% water agar in Petri dishes. The exposed ends of the explants were inoculated with 2 μL of bacterial suspensions (108 CFU/ml). Three explants were used as a control, one untreated, one treated with sterile distilled water and one treated with A. tumefaciens. The appearance of necrosis was recorded after 72 h. Inoculation technique: Two days before inoculation, the strain were cultured onto potato dextrose agar (PDA) (Difco) and grown at 28°C. Colonies from PDA were suspended at about 109 colony forming units (cfu) per ml in sterile de-ionized water (SDW). 10 μL of bacterial suspension were used to 1 mm diameter holes drilled through shoot nodes with typically 4 sites in green tissue. Inoculated sites were covered with parafilm and plants were grown in the greenhouse. After five months, inoculated sites were scored for gall formation.
In the second set, inoculations were made by adding 40 ml of the suspension of two strains of bacteria (with the same concentration) around the root.
Measurements of biochemical parameters
Chlorophyll and carotenoids: for extraction of chlorophyll, 0.1gr fresh leave placed in mortar. Then 10 ml of 80% acetone added to sample and ground with mortar and pestle. The extraction was centrifuged 10 min in 2700 rpm. Supernatant was transferred to cuvette and absorbance was read in a UV-VIS spectrophotometer at 3 wavelengths, 470, 647,663. The concentrations of chlorophyll a and b were calculated based on following equations:
1. Chla: 12.25A663-2.79A647
2. Chlb: 21.5A647- 5.1A663
3. Car: (1000A470-1.82Chla-85.02Chlb)/198
Soluble carbohydrate: 0.1 g of leaves (dry weight) was used for soluble carbohydrates determination by phenol-sulfuric acid method [11].
Anthocyanin: for extraction of anthocyanin, 0.1 g fresh leave with 10 ml methanol and HCL (99/1) ground with mortar and pestle. The extraction was centrifuged 5 min in 5000 rpm. Supernatant was transferred to cuvette and absorbance was read in a UV-VIS spectrophotometer at 550 nm wavelengths. The concentrations of anthocyanin were calculated with the following equations:
4. Concentrations of anthocyanin = the absorbance/33000
Statistical analysis
Data was subjected to analysis of variance and means were separated using Fisherʼs protected LSD. The statement regarding statistical significance indicates that means were different at the P=0.05 level.
Results
Tumorigenicity
In this study the effect of bacterial isolates to induce galls was studied. Figure 1 shows gall formation on tomato and callus formation on carrot.
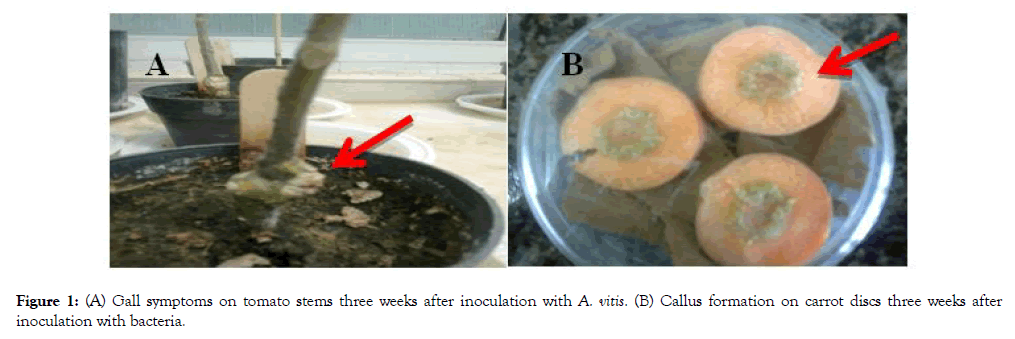
Figure 1: (A) Gall symptoms on tomato stems three weeks after inoculation with A. vitis. (B) Callus formation on carrot discs three weeks after inoculation with bacteria.
Determination of phenotypical characterization Typical Agrobacterium colonies from tumors and plant sap were isolated on NA media. They appeared to be convex, smooth with distinct edges. Table 1 shows the phenotypical characteristics of these isolate.
Table 1: Phonotypical characteristics of Agrobacterium isolates.
| Isolate(1) | Isolate (2) | A. tumefaciens (Control) | Test |
|---|---|---|---|
| - | - | - | Gram reaction |
| Yellow | Yellow | Yellow | YDC pigment |
| - | - | - | Fluorescent pigment |
| O | O | O | O/F |
| + | + | + | Catalase |
| - | - | - | Gelatin hydrolyses |
| + + | + + | - - | Utilization of Malonic AcidL-Tartaric Acid |
| - | - | + | Motility at PH 7 |
| + | + | - | Pectolytic at PH 4.5 |
+: Positive reaction or growth
-: Negative reaction or growth
Callus formation
The size of explants, the ratio of hormones (or bacteria) and the genotype are major factors for the callus formation of grapevines. In this study, genotype of grape was checked. According to the result, Rish baba and Qazvin Sefid-e- Keshmeshi have highest rate of callus in bacteria and hormone treatment (Figures 2 and 3).
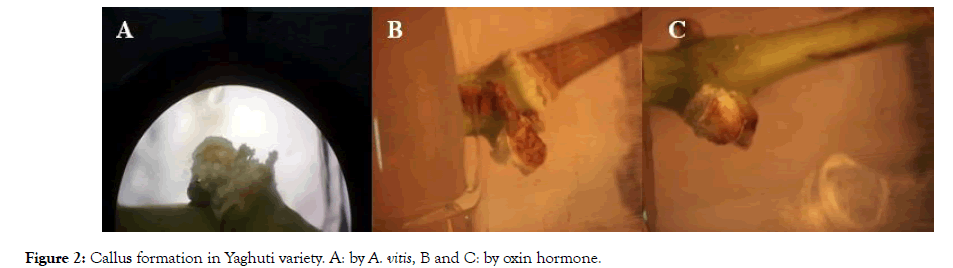
Figure 2: Callus formation in Yaghuti variety. A: by A. vitis, B and C: by oxin hormone.
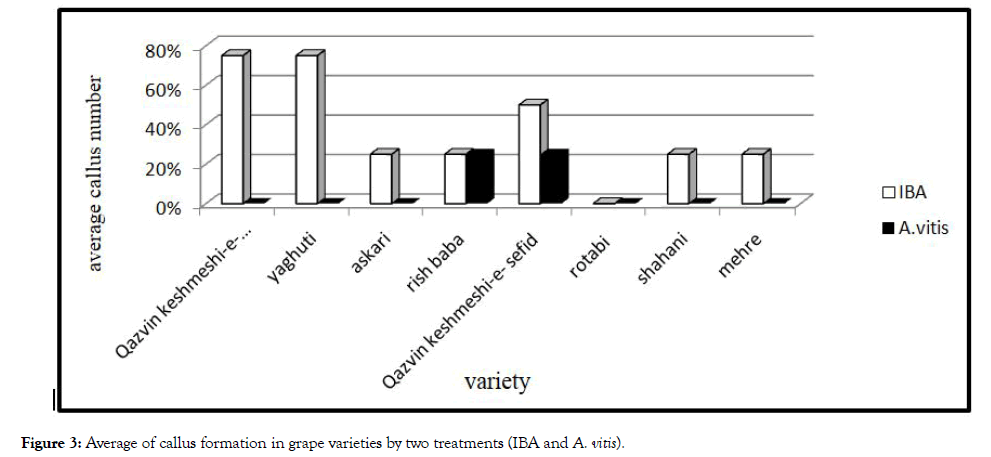
Figure 3: Average of callus formation in grape varieties by two treatments (IBA and A. vitis).
Ability to induction grape necrosis
These isolates were identified that caused necrosis on grape explants (Figure 4). Severity of necrosis differed between varieties which bacterium caused a black necrosis with ooze within 48 h in Shahani and Yaghuti. By streaking cut ends of the explants on NA medium, the standard colonies of A. vitis readily appeared after 2 days (Table 2).

Figure 4: Necrosis on grape explants (Yaghuti) resulted from artificial inoculation by A. vitis.
Table 2: Mean comparison* of necrosis depth caused by crown gall agent (A. vitis).
| Necrosis depth (mm) | Treatment | Variety |
|---|---|---|
| 1.37abc | A. vitis | Askari |
| 1.50ab | A. tumefaciens | Askari |
| 0.75defg | sterile distilled water | Askari |
| 1.00bcde | Control | Askari |
| 0.50efgh | A. vitis | Qazvin Ghermez-e- Keshmeshi |
| 0.62efgh | A. tumefaciens | Qazvin Ghermez-e- Keshmeshi |
| 0.75h | sterile distilled water | Qazvin Ghermez-e- Keshmeshi |
| 0.15h | Control | Qazvin Ghermez-e- Keshmeshi |
| 0.62efgh | A. vitis | Qazvin Sefid-e- Keshmeshi |
| 0.75defg | A. tumefaciens | Qazvin Sefid-e- Keshmeshi |
| 0.37fgh | sterile distilled water | Qazvin Sefid-e- Keshmeshi |
| 0.25gh | Control | Qazvin Sefid-e- Keshmeshi |
| 1.75a | A. vitis | Mehre |
| 1.25abcd | A. tumefaciens | Mehre |
| 0.62efgh | sterile distilled water | Mehre |
| 0.50efgh | Control | Mehre |
| 1.37abc | A. vitis | Rish baba |
| 1.5ab | A. tumefaciens | Rish baba |
| 0.52efgh | sterile distilled water | Rish baba |
| 0.15h | Control | Rish baba |
| 1.00bcde | A. vitis | Rotabi |
| 0.87cdef | A. tumefaciens | Rotabi |
| 0.27gh | sterile distilled water | Rotabi |
| 0.15h | Control | Rotabi |
| 1.37abc | A. vitis | Shahani |
| 0.87cdef | A. tumefaciens | Shahani |
| 0.37fgh | sterile distilled water | Shahani |
| 0.25gh | Control | Shahani |
| 1.62a | A. vitis | Yaghuti |
| 0.75defg | A. tumefaciens | Yaghuti |
| 0.37fgh | sterile distilled water | Yaghuti |
| 0.17h | Control | Yaghuti |
*Means with the same letters in each column are not significantly different at 5% level according to LSD test.
Plant growth indices
Analysis variance of bacterium action on plant growth indices showed a significant effect (Figure 5). This bacterium reduced the growth vigor, especially stem length. Highest effect of this bacteria observed on Shahani variety. Tumors originate from dividing plant cells, e.g. from cambium. Thus the cambial region becomes unable to differentiate into efficient phloem and xylem elements leading to deficient nutrient transport, and grapevine decline, and vine death and yield reduce [12].
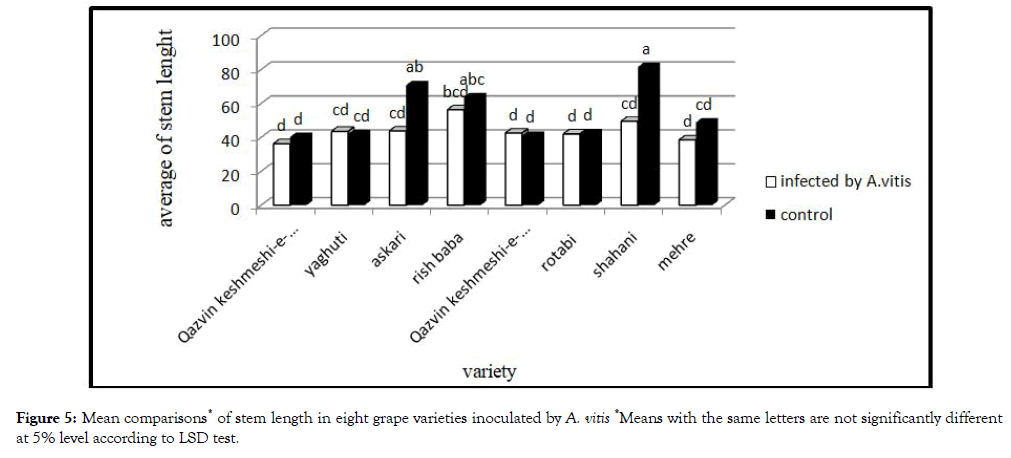
Figure 5: Mean comparisons* of stem length in eight grape varieties inoculated by A. vitis *Means with the same letters are not significantly different at 5% level according to LSD test.
Plant biochemical indices
Chlorophyll and carotenoids: Mean comparisonof effect of bacterium on plant photosynthesis pigments showed different action (Figure 6). According to these results, bacterium could reduce photosynthesis pigments, but most reduction observed by Yaghuti and Rish baba.
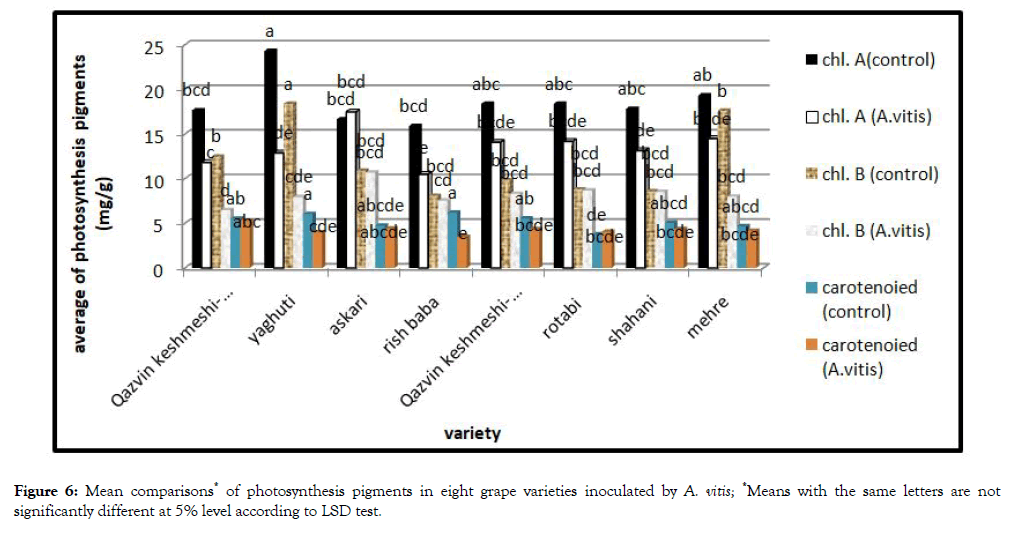
Figure 6: Mean comparisons* of photosynthesis pigments in eight grape varieties inoculated by A. vitis; *Means with the same letters are not significantly different at 5% level according to LSD test.
According to literature, drought is one of the leading environmental stresses decreasing plant photosynthesis pigments, then growth and productivity [13]. Gall formation on roots and shoots, leads todisturbance of absorption and transmission of water and finally lack of water and water stress in the plant occurs.
Soluble carbohydrate: The result of this study showed that the soluble carbohydrate in the leaves of treatment plants significantly increased. The greatest increase was observed in the Shahani and Rotabi (Figure 7).
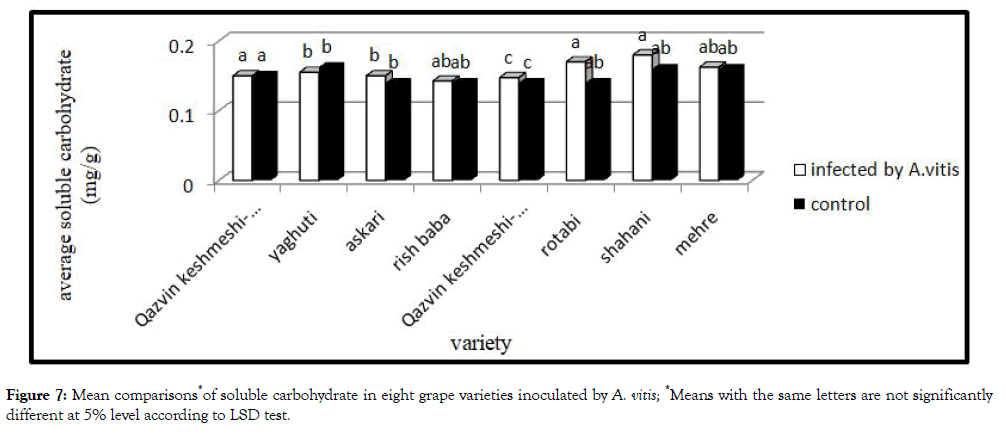
Figure 7: Mean comparisons*of soluble carbohydrate in eight grape varieties inoculated by A. vitis; *Means with the same letters are not significantly different at 5% level according to LSD test.
Studies show that stress increases soluble carbohydrate. Accumulation of Soluble carbohydrate in these condition helps to regulate intercellular osmolarity [14].
Anthocyanin: Mean comparison of effect of bacterium on plant anthocyanin showed different action (Figure 8). Bacteria increased anthocyanin content in the plant. Treatment were infected with bacteria have more anthocyanin, while highest significant increase in Shahani and Rotabi was observed.
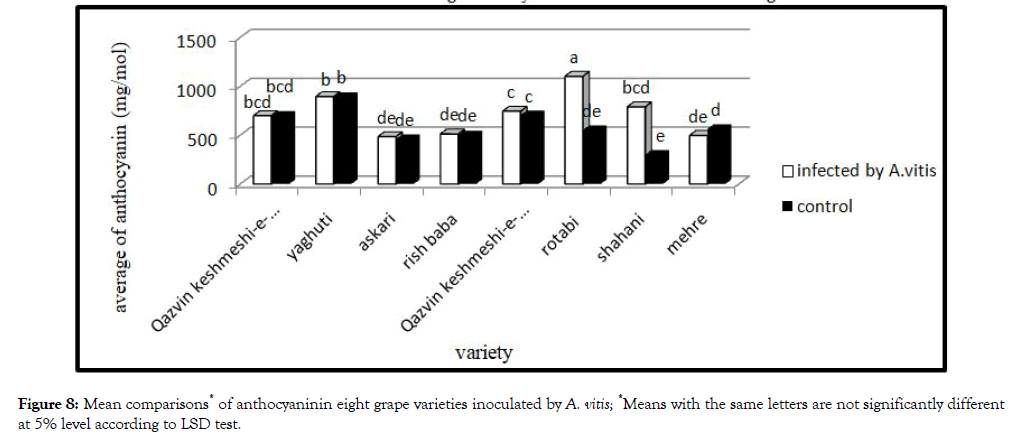
Figure 8: Mean comparisons* of anthocyaninin eight grape varieties inoculated by A. vitis; *Means with the same letters are not significantly different at 5% level according to LSD test.
Accumulation of anthocyanin in leaves, shoots and root by several environmental stress such as drought and wound has been reported earlier [15]. In this study bacterial infection, drought and wound are most important factors increasing the amount of anthocyanin.
Crown gall agent pathogenicity indices
Analysis variance of bacterium pathogenicity indices showed no significant different between varieties (Table 3). Yaghuti has highest gall between eight varieties (Figure 9).
Table 3: Analysis variance of crown gall agent pathogenicity indices on eight infected grape varieties.
| Sources of Variation | Df | MS (Mean Square) |
|---|---|---|
| Gall number | ||
| Variety | 7 | 7.45n.s |
| Error | 24 | 4.71n.s |
| CV% | 27.44 |
n.s: No Significant at the 0.05 level
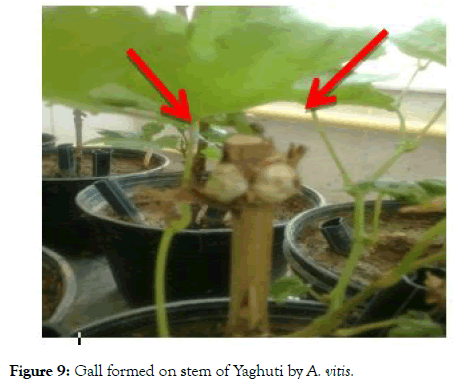
Figure 9: Gall formed on stem of Yaghuti by A. vitis.
Tumors are a proliferation and agglomeration of tissue caused by the toxins secreted by bacteria. Cytological aspect of tumor tissue present within a tumor is much like that found in humans and animals. In a cross-section of tumor cells large, giant cells where are surrounded by normal-sized cells were observed. Many cells are polinucleate and retain strong dyes. In the tumor was found an accumulation of organo-forming substances (ex: β- indol acetic acid) and organic acids (malic, citric, oxalic, etc.) [16].
Crown gall agent pathogenicity indices (second set of experiment)
Analysis variance of bacterium pathogenicity indices showed significant different between eight grape varieties. The number of gall in grape varieties was different. Yaghuti hadthe largest number of gall were infected with both isolates. Thus, Yaghuti was the most sensitive variety in this study. On the other hand, Shahani had the lowest number of gall which can be due to its insensitivity to growth hormone (Figures 10 and 11).
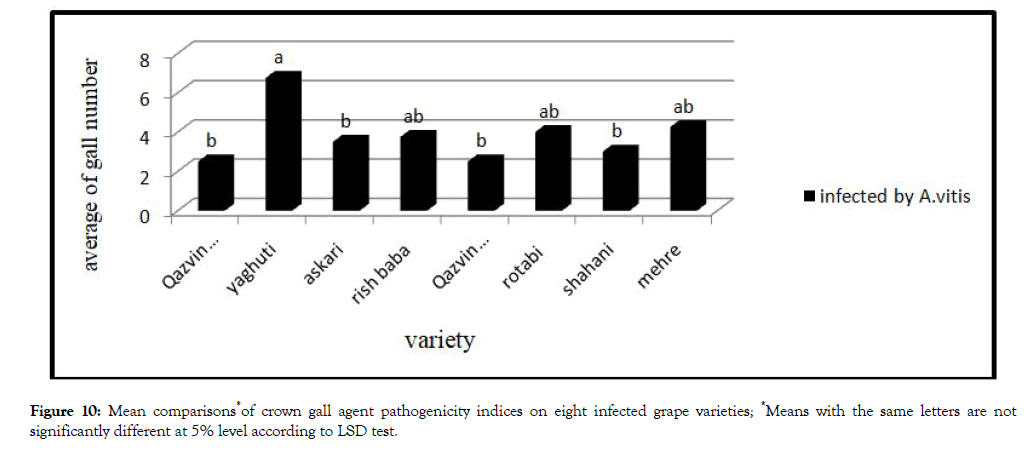
Figure 10: Mean comparisons*of crown gall agent pathogenicity indices on eight infected grape varieties; *Means with the same letters are not significantly different at 5% level according to LSD test.
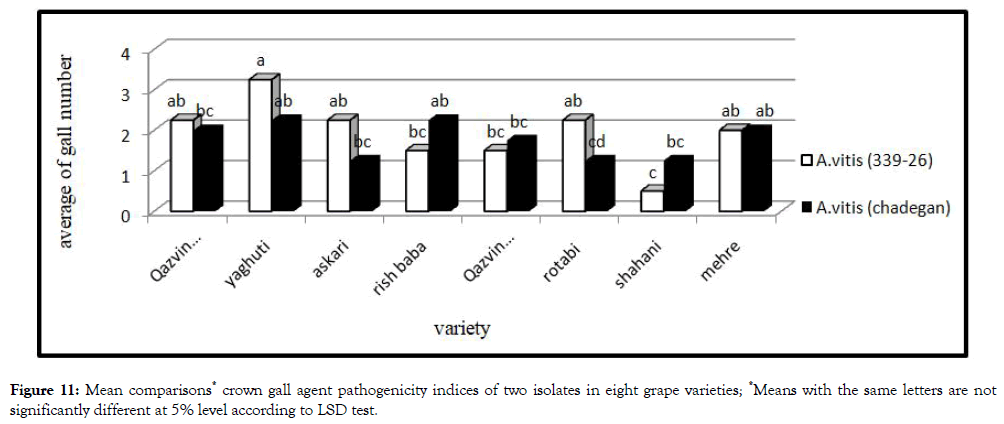
Figure 11: Mean comparisons* crown gall agent pathogenicity indices of two isolates in eight grape varieties; *Means with the same letters are not significantly different at 5% level according to LSD test.
Discussion
In this study, no variety was found to be immune to crown gall, while response of different varieties to inoculation with A. vitis varied widely. In all grape varieties, gall formation leads to disruption of the vascular system. That finally led to diminishment growing plant. Highest reduction of growth indices observed on Shahani. The plant infection with bacteria (A.vitis) showed an influence on biochemical parameters. Shahani is most sensitive variety in biochemical parameters. Also callus formation and necrosis depth showed that Yaghuti is the most sensitive variety to A. vitis. Shahani was sensitive to A. vitis, and bacterial infection made reduction effect on growth indices and adequate to biochemical indices in this variety. While the number of gall formed was lowest among another varieties, that according to result of callus formation, Shahani is not susceptible to growth regulator hormones (auxin and cytokinin).
Conclusion
Finally according to these results, Shahani, Yaghuti and Rotabi are most sensitive varieties to crown gall agent. Rootstock resistance to crown gall may be important in preventing passage of soil A. vitis in to susceptible scions. However, other factors are probably involves in the development of systematic infections. Also freeze injury is an important factor in systemic A. vitis development in the field, and use of resistance rootstock to Freezing is an effective way to control of this dieses. When resistant rootstocks form galls, populations of A. vitis in the nodal tissues remain much lower than in susceptible and this may affect the extent of endophytic colonization. However, it is importance to note that high populations of A. vitis can survive at inoculated sites of both crown gall-resistant and susceptible vitis rootstocks for more than a year without inducing visible crown gall symptoms.
REFERENCES
- Burr JT, Bazzi C, Sule S, Otten, L. Biology of Agrobacterium vitis and development of disease control strategies. J Plant Disease. 1998;82(12):1288-1297.
- Schroth MN, McCain AH, Foott JH, Huisman OC. Reduction in yield and vigor of grapevine caused by crown gall disease. Plant Dis. 1988;72:241-246.
- Dhanvantari BN. Etiology of grape crown gall in Ontario. Can J Bot. 1983;61:2641-2646.
- Burr TJ, KatzBH. Grapevine cuttings as potential sites of survival and means of dissemination of Agrobacterium tumefaciens. Plant Dis. 1984;68:976-978.
- Ophel K, Kerr A. Agrobacterium vitis sp nov. for strains of Agrobacterium biovar 3 from grapevines . Intern J Syst Bacteriol. 1990;40:236-241.
- Bishop AL, Katz BH, Burr TJ. Infection of grapevines by soil borne Agrobacterium tumefaciens biovar 3 and population dynamics in host and nonhost rhizospheres. Phytopathol. 1988;78:945-948.
- Szegidi E, Korbuly J, Koleda I. Crown gall resistance in East-Asian vitis species and in their V. vinifera hybrids. Journal of Grapevine Research. 1984;23:21-26.
- Stover EW, Burr TJ, Swarts HJ. Transformation of crown gall resistant and susceptible vitis genotype by Agrobacterium vitis. J vitis. 1996;35(1):29-33.
- Roy MA, Sasser M. A medium selective for Agrobacterium tumefaciens biotype 3. (Abstr.). Phytopathol. 1983;73:800-810.
- Liao CH, Heberlain GT. A method for the transfer of tumorigenicity between strains of Agrobacterium tumefaciens in carrot root disks. Phytopathol. 1978;68:135-137.
- Kochert G. Carbohydrate determination by the phenol sulfuric acid method. In: Helebust, JA, Craigie JS(Ed): Hand book of physiological methods. Cambridge unir. Press, Cambridge. 1978; 96-97.
- Filo A, Sabbatini P, Sundin GW, Zabadal TJ, Safir GR, Cousins PS. Grapevine Crown Gall Suppression Using Biological Control and Genetic Engineering: A Review of Recent Research. Vitic. 2013;64(1):1-14.
- Ghaderi N, Siosemardeh A. Response to Drought Stress of Two Strawberry Cultivars (cv. Kurdistan and Selva). Hort. Environ. Biotechnol. 2011;52(1):6-12.
- Sadras VO, Milroy SP. Soil water thresholds for the responses of leaf expansion and gas exchanges. A review. Field crops Res. 1996;47:256-266.
- Nagira Y, Ikegami K, Koshiba T, Ozeki Y. Effect of ABA upon anthocyanin synthesis in regenerated to renia shoots. J Plant Res 2006;19:137-144.
- DianaV, Dejeu L. Crown gall (Agrobacterium spp.) and vitis. J. Horticulture, Forestry and Biotechnology. 2011;15(1):130-138.
Citation: Fadaei AA, Fathi SH (2020) A Study on the Reaction of Some Grapevine Varieties to Crown Gall Agent (Agrobacterium vitis). Plant Pathol Microbiol. 11:498.doi: 10.35248/2157-7471.20.11.498.
Copyright: 2020 Fadaei AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.