Indexed In
- Open J Gate
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2024) Volume 13, Issue 3
A Study on the Prevalence of E. coli in the Urinary Tract Infection and the Risk Factors Associated with It
Rajrupa Ghosh* and Shiblee SarwarReceived: 16-Aug-2024, Manuscript No. CMO-24-26760; Editor assigned: 19-Aug-2024, Pre QC No. CMO-24-26760 (PQ); Reviewed: 02-Sep-2024, QC No. CMO-24-26760; Revised: 09-Sep-2024, Manuscript No. CMO-24-26760 (R); Published: 16-Sep-2024, DOI: 10.35248/2329-8847.24.13.404
Abstract
Millions of people worldwide suffer from infections of the Urinary Tract Infections (UTIs) brought on by the bacteria Escherichia coli (E. coli), which represents a substantial global health burden. It is essential to comprehend the epidemiology and risk factors that are linked to these illnesses in order to develop appropriate therapy and preventative techniques. The frequency, distribution patterns, and risk factors of UTIs linked to E. coli are examined in this study, with particular attention paid to age, gender, underlying medical problems, and opiate usage. The study intends to improve knowledge of UTI the pathogenesis of clinical symptoms, diagnostic techniques, treatment strategies, and preventive measures by analysis of clinical information, statistical models, and previous research. The results highlight how important it is to treat E. coli UTIs systemically, using alternative treatments, and with caution when using antibiotics. The study emphasises the necessity of ongoing efforts to reduce the incidence of UTIs caused by E. coli by means of focused interventions, policy formulation, and public health campaigns. In the end, this dissertation advances knowledge, directs future research paths, and enhances clinical results in the treatment of E. coli caused UTIs.
Keywords
UTI; Infection; Pathogenesis; Antibiotics; Urine; Gastrointestinal tract
Introduction
Millions of people worldwide suffer from common bacterial illnesses known as urinary tract infections, or UTIs. Of all the microorganisms that cause urinary tract infections, Escherichia coli, or E. coli, is one of the main offenders. This bacterium can cause an infection in the urinary system after ascending from the gastrointestinal tract. Effective prevention and management of UTIs need knowledge of the prevalence of E. coli and the identification of risk factors linked with the infection [1,2].
The epidemiology of E. coli related UTIs is examined in this dissertation, with particular attention paid to variables including age, gender, underlying medical problems, and opioid use. We want to clarify the incidence rates, distribution patterns, and probable risk factors related to urinary tract E. coli infections by assessing clinical data, statistical models, and previously published research [2].
The results of our inquiry will be useful in directing methods for prevention, early identification, and focused therapies for researchers, policymakers, and healthcare practitioners. Encountering E. coli and its effects on urinary tract health: Let's go on this scientific expedition to solve the puzzles [1].
Global perspective
About 80% of UTIs worldwide are caused by E. coli. People of all ages can be affected by these illnesses, which can happen in both community and hospital. Over the past several decades, the incidence of urinary tract infections has been gradually increased. We have to know of its incidence and related causes [1,2].
Indian scenario
UTIs are a serious health concern in India. A recent study that examined 8072 samples found that UTIs were common between males and females. Remarkably, E. coli caused a significant percentage of cases and was the most frequently isolated bacterium. According to the gender distribution, women between the ages of 15 and 44 were shown to be more vulnerable to UTIs [3,4].
Materials and Methods
Materials
These are the materials used for the study on E. coli prevalence in Urinary Tract Infections (UTIs):
Urine sample collection materials: A) Sterile urine collection containers, B) Sterile gloves.
Laboratory equipment: A) Microscope, B) Incubator, C) Culture media plates (e.g., MacConkey agar, blood agar), D) Antibiotic susceptibility testing materials (e.g., antibiotic discs).
Chemicals and reagents: A) Urinalysis dipsticks or reagent strips, B) Staining reagents (e.g., Gram stain), C) Culture media components.
Antibiotics: Common antibiotics used for UTIs caused by E. coli (e.g., trimethoprim-sulfamethoxazole, nitrofurantoin, fluoroquinolones).
Safety equipment: A) Lab coats, B) Safety goggles, C) Biohazard waste disposal containers.
Methods
These are the methods used for the study on E. coli prevalence in Urinary Tract Infections (UTIs):
Sample collection: Following the steps below is essential for accurate results:
• Obtain urine samples from patients suspected of having UTIs caused by E. coli using sterile techniques.
• Sample is collected from, Netaji Subhas Chandra Bose Cancer Hospital, 3081, Nayabad Ave, New Garia, Pancha Sayar, Kolkata, West Bengal 700094.
• Urine samples are collected in a number of stages that are meticulously carried out to guarantee precision and dependability while doing tests on them. Initially, the medical professional gives the patient a sterile container. Then, the medical staff provides detailed instructions on how to collect samples properly. Assuring that the procedure is performed in a clean and sanitary way, the patient follows these directions and collects the specimen of urine in the specified container. To stop contamination or manipulation, the sample is tightly sealed once it has been gathered. When the sample is finally sent to the lab for examination, it is tagged with pertinent details including the patient's name, the date, and the time of collection [2,5,6].
• Throughout this whole process, strictly followed the protocols and guidelines to maintain the integrity and validity of the urine sample for diagnostic purposes.
Urinalysis: To accurately assess potential infections, the following procedures should be followed:
• Perform urinalysis using dipstick tests or reagent strips to detect indicators of infection such as leukocytes, nitrites, and blood [7,8].
• Choose an appropriate urinalysis dipstick containing reactive strips for the parameters to be tested.
• Carefully dip the reactive portion of the dipstick into the urine sample, ensuring that all reactive pads are fully submerged into the urine sample.
• Allow the dipstick to remain immersed in the urine for the specified reaction time, typically a few seconds to a minute, depending on the manufacturer's instructions.
• During this time, the reactive pads on the dipstick will undergo chemical reactions with specific components present in the urine sample.
• After the designated reaction time, remove the dipstick from the urine sample and observe any colour changes on the reactive pads.
• Note the intensity and hue of each colour change, as these correspond to the concentration or presence of the substances being tested.
• Immediately compare the colours on the reactive pads to the standardized colour chart provided by the manufacturer.
• Match the observed colours to those on the chart to determine the concentration or level of each tested parameter in the urine samples.
• Record the results of the urinalysis, including the interpretation of dipstick reactions, in the appropriate documentation or laboratory report.
• Ensure accurate labelling and documentation of the patient's information and test findings.
Microscopy
Microscopy is the technique of using microscopes to view objects and areas of objects that cannot be seen with the naked eye. It allows scientists to study the fine details of cells, tissues, and other small structures. Common types include optical, electron, and fluorescence microscopy, each offering unique magnification and resolution abilities.
Materials required: These are the following materials used in microscopy:
• Microscope
• Urine sample from the patient
• Centrifuge
• Microscope Slides
• Coverslips
• Pipettes
• Gloves.
Steps: These are the following steps used in microscopy:
• Make sure that the microscope is clean.
• Label the microscope slides with the patient's information such as age, sex, etc.
• Put on laboratory gloves to maintain aseptic conditions.
• Obtain a fresh urine sample from the patient, preferably a midstream clean-catch sample.
• Mix the urine sample gently to ensure uniformity before further processing.
• After mixing take the sample in centrifuge tube and centrifuge the sample at a low speed (usually around 2000 rpm) for 5-10 min.
• After that, carefully remove the supernatant urine and retain the sediment at the bottom of the tube.
• Using a pipette, take a small amount of the sediment from the centrifuged urine sample onto a clean microscope slide.
• Cover the prepared slide with a coverslip and gently press down it to remove any air bubbles [9-11].
• Place the prepared slide on the microscope stage and adjust the magnification to the lowest setting (usually 10x objective lens).
• Scan the entire area of the slide systematically, observing for any structures or abnormalities.
• Once an area of interest is identified, increase the magnification (usually to 40x or 100x objective lens) for detailed examination.
• Identify and characterize various elements present in the urine sediment, including: RBC, WBC, Epithelial Cells, Cast (Hyaline, Granular, Cellular), Crystals.
Urinalysis findings: Urinalysis findings commonly include the presence of leukocytes, nitrites, blood, and bacteria, indicating an active urinary tract infection (Figure 1).
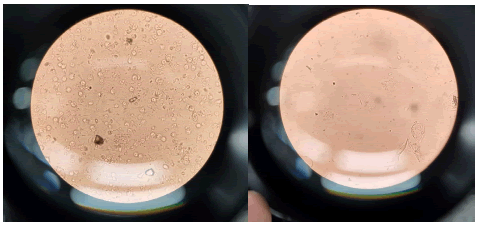
Figure 1: Urine slide representation.
Culture and identification
• Inoculate urine samples onto appropriate culture media plates and incubate them to encourage bacterial growth.
• Using an inoculating loop, to streak or spread a portion of the urine sample onto appropriate culture media. (MacConkey agar for Gram-negative bacteria).
• After inoculation the inoculated culture plates aerobically incubated at 35°C-37°C for 24-48 h.
• Identify E. coli colonies based on characteristic morphology, the colonies on MacConkey agar are pink in colour due to lactose fermentation [12-14].
E. coli colony count: The colony count of E. coli in urine samples varies among patients, ranging from 103 to 107 CFU/ml.
Confirmation of E. coli: Colonies that resemble E. coli (such as pinkish or lactose-fermenting colonies) are further tested. MacConkey agar is differential and selective media which is used to isolate only gram-negative bacteria and differentiate it from lactose fermented and non-lactose fermented bacteria. E. coli is gram negative and lactose fermenting bacteria [12,15,16].
Biochemical test: Laboratory methods for identifying and classifying bacteria according to their biochemical characteristics are called microbiological biochemical assays. These assays use the distinct metabolic properties of various bacteria to distinguish between strains or species. Microbiologists can make inferences about the identification and traits of the organisms they are studying by watching how the microbes use certain nutrients or substrates, make particular enzymes, or produce metabolic byproducts. Here I perform the test Indole, Catalase and Methyl Red to confirm that the cultured bacteria are E. coli (Figure 2).
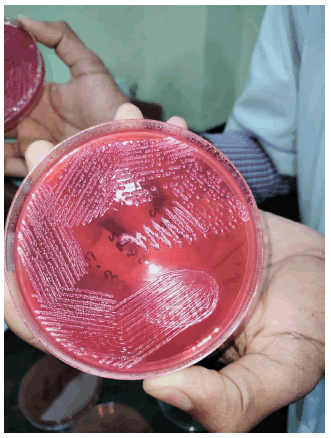
Figure 2: E. coli on MacConkey agar.
Indole test
The indole test is a biochemical assay used to determine an organism's ability to produce indole from the amino acid tryptophan. Bacteria that can break down tryptophan produce indole, which reacts with Kovac’s reagent to form a red or pink color. This test is commonly used to identify bacteria, especially in differentiating Escherichia coli from other enteric bacteria (Figure 3).

Figure 3: Indole positive representation.
Purpose: To detect the production of indole from tryptophan.
Principle: E. coli possesses the enzyme tryptophanase, which breaks down tryptophan into indole, pyruvate, and ammonia.
Procedure: The following steps are used to obtain accurate results:
• A small loopful of the bacterial colony from the urine culture is inoculated into a tube containing tryptone broth (a medium rich in tryptophan.
• The tube is incubated at 37°C for 24 h.
• After incubation, Kovac’s reagent is added to the tube.
• A positive result is indicated by the appearance of a red colour at the surface of the medium [17-19].
Interpretation: Indole positive
Positive indole test: E. coli produces indole (red colour).
Negative indole test: Other bacteria may not produce indole.
Methyl Red (MR) test
The Methyl Red (MR) test is a biochemical test used to detect the production of stable acids from glucose fermentation by bacteria. In this test, the pH indicator methyl red is added to the bacterial culture, turning red in acidic conditions (pH below 4.4), indicating a positive result. It is commonly used to differentiate between mixed acid fermenters, such as Escherichia coli, and bacteria that produce neutral end products.
Purpose: To assess the ability of the bacterium to perform mixed acid fermentation.
Principle: E. coli produces large amounts of acid during glucose fermentation.
Procedure: 1) A portion of the bacterial culture is inoculated into MR-VP (methyl red-Voges-Proskauer) broth, 2) The tube is incubated at 37°C for 48 hours, 3) After incubation, methyl red indicator is added, 4) A positive result is indicated by a red colour (low pH due to acid production) [20-22].
Interpretation: Methyl Red (MR) Positive
Positive MR test: E. coli produces acid (red colour).
Negative MR test: Other bacteria may not produce sufficient acid.
Catalase test
• The catalase test is used to identify whether a bacterial species produces the enzyme catalase or not.
• Catalase is an enzyme that breaks down hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2).
• The reaction can be represented as follows:
2H2O2→2H2O+O2
• The quick release of oxygen bubbles occurs when a colony of bacteria comes into contact to hydrogen peroxide in the presence of catalase.
• To prepare the bacterial suspension, we have started with an agar plate containing a pure culture of Escherichia coli. A tiny bit of the bacterial culture is transferred using a sterile loop or swab into a test tube that has a small amount of sterile saline solution (0.85% NaCl) in it (around 1-2 ml). The culture is mixed well to guarantee uniform dissemination.
Labelling and organisation: Then we have written the name or code of the bacterium (E. coli) that is being tested on the test tube along with any other pertinent information, including the date. Test tubes are placed in a rack to make handling them easier [22,23].
Adding hydrogen peroxide: A few drops (about three to four) of a 3% hydrogen peroxide solution is transferred straight into the test tube containing the bacterial suspension.
Observation: Then we have watched for the prompt response. If E. coli develops catalase, the breakdown of the hydrogen peroxide will result in the fast production of oxygen gas bubbles. A catalase test that is positive is indicated by the presence of bubbles.
Interpretation: A positive test indicates that E. coli generates catalase if bubbles are seen in a short period of time (Table 1).
| Patient ID | Age | Gender | Symptoms | Urinalysis findings | E. coli colony count (CFU/mL) | Antibiotic susceptibility | Treatment outcome |
|---|---|---|---|---|---|---|---|
| 1 | 35 | Female | Dysuria, frequency, urgency | Positive for leukocytes, nitrites | 10^5 | Susceptible to trimethoprim-sulfamethoxazole, nitrofurantoin | Symptoms resolved after 3 days of trimethoprim-sulfamethoxazole |
| 2 | 60 | Male | Fever, flank pain, cloudy urine | Positive for leukocytes, blood | 10^6 | Resistant to ciprofloxacin, susceptible to nitrofurantoin | Required hospitalization and intravenous antibiotics due to pyelonephritis |
| 3 | 25 | Female | Lower abdominal pain, foul-smelling urine | Positive for leukocytes, bacteria | 10^4 | Susceptible to ceftriaxone, resistant to trimethoprim-sulfamethoxazole | Symptoms improved after 5 days of ceftriaxone |
| 4 | 45 | Male | Burning sensation during urination | Positive for leukocytes | 10^3 | Intermediate to nitrofurantoin, susceptible to fosfomycin | Symptoms resolved after 7 days of fosfomycin |
| 5 | 55 | Female | Hematuria, fever | Positive for leukocytes, nitrites, blood | 10^7 | Resistant to nitrofurantoin, susceptible to levofloxacin | Developed acute kidney injury, required intensive care |
Table 1: Observation table of males and females suffered with Urinary Tract infection.
Antibiotic susceptibility testing
To find the best antibiotics for therapy, do antibiotic susceptibility testing using techniques like the Kirby-Bauer disc diffusion method.
Kirby-Bauer disc diffusion method
In clinical microbiology, the Kirby-Bauer disc diffusion method- also known to as the disc diffusion test [24-26] or resistance to antibiotic testing is commonly used technique for ascertaining the antibiotic susceptibility of microorganisms. This is a summary of the steps involved in this testing:
Preparation of bacterial culture: Getting a pure culture, such as using the E. coli isolate from a urine sample. After that the bacteria are cultivated on Mueller Hinton agar, to create a consistent bacterial growth.
Antibiotic disc placement: Sterile antibiotic disks impregnated with specific concentrations of antibiotics are placed equally distributed on the surface of the agar plate. Each disk has a different antibiotic.
Incubation: After the disc placed on the plate, the plate must have to incubate for an amount of time again like 16-18 h in 37°C. In this period the bacteria proliferate and manifest as visible lawn on the agar surface.
Measurement of Inhibition zone: After the incubation measured the zone of inhibition which is actually a clear region that shows surrounding the disc where the bacterial growth is suppressed are looked for on the plate. One can use the automated tool or calibrated ruler to measure the zone of inhibition.
Interpretation: The diameter of the zone of inhibition is compared to established interpretative criteria provided by organizations such as the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST). This makes easier to categorise the bacteria as sensitive, intermediate or resistance to the used antibiotic (Figure 4).
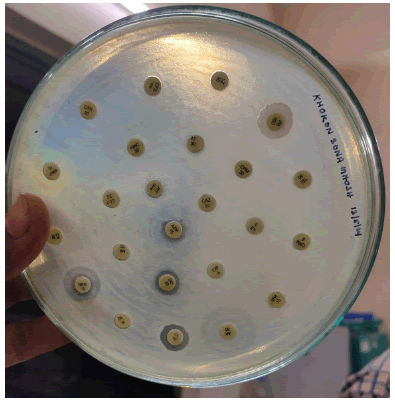
Figure 4: Antibiotic susceptibility representation.
Results
The table indicates the susceptibility of E. coli isolates to different antibiotics commonly used for UTI treatment.
Susceptibility testing results show variability in susceptibility patterns among the isolates. Some isolates are susceptible to certain antibiotics, indicating that these antibiotics are effective against those strains of E. coli. Others may show resistance or intermediate susceptibility to certain antibiotics, suggesting reduced effectiveness or potential treatment challenges.
Trimethoprim-sulfamethoxazole and nitrofurantoin appear to be effective against a majority of the E. coli isolates tested, as they are labeled as “susceptible.”
Ciprofloxacin, on the other hand, shows resistance in some isolates, indicating reduced effectiveness.
Other antibiotics such as ceftriaxone and fosfomycin also demonstrate variable susceptibility patterns among the isolates.
Intermediate susceptibility to nitrofurantoin is observed in one isolate, indicating potential treatment challenges or the need for higher doses.
Discussion
Age distribution
The bulk of patients in this dataset are adult patients; there is no representation of paediatric or elderly patients. Patients with E. coli- caused UTIs range in age from 15 to 60 yrs old. This distribution indicates that E. coli-related UTIs can impact people of all ages, although adults are more likely to have them (Figure 5).
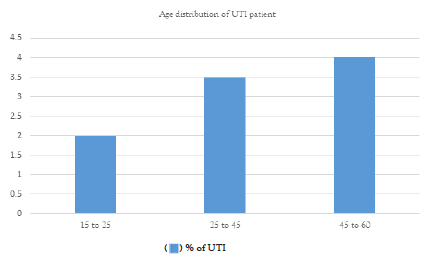
Figure 5: Age distribution chart of UTI patients.
Gender distribution
• The gender distribution shows that females are most experiencing UTI caused by E. coli in this sample.
• The dataset includes both male and female patients. This research supports the widely held belief that Urinary Tract Infections (UTIs) can afflict people of any gender, however women often have a greater frequency of UTIs overall because of anatomical variations (Figure 6).
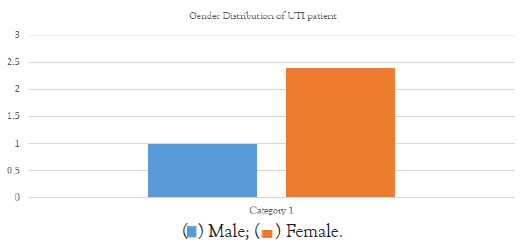
Figure 6: Gender distribution chart of UTI patients.
Symptoms
These are the following symptoms for UTIs:
Frequent and painful urination: Patients may experience a persistent urge to urinate, often accompanied by a burning sensation during urination. This symptom is known as dysuria [2].
Cloudy or foul-smelling urine: Urine infected with E. coli may appear cloudy or have an unpleasant odour, indicative of the presence of bacteria in the urinary tract.
Lower abdominal pain or discomfort: Some individuals with E. coli-related UTIs may experience discomfort or pressure in the lower abdomen, often localized around the bladder area [27-29].
Haematuria: Haematuria refers to the presence of blood in the urine. In UTIs caused by E. coli, patients may notice pink, red, or cola-coloured urine, signalling possible inflammation or damage to the urinary tract lining.
Fever and malaise: In more severe cases or when the infection spreads to the kidneys, patients may develop fever, chills, and overall feelings of malaise or fatigue as the body mounts an immune response to the bacterial invasion [11].
Treatment outcome
The results of treatment for each patient are different, some get better with antibiotics while some get worse and need to be hospitalised or suffer from consequence including kidney damage [28].
Examine if treating E. coli related UTIs with antibiotic is successful. Analyse the effectiveness of several antibiotic regimens that are often used to treat UTIs caused by E. coli, taking into account variable including recurrence rate, microbial eradication and symptoms resolution. Examine how antibiotic resistance effect treatment results as well as the necessity of using different treatment approaches.
Look at the link between treatment results, especially hospitalisation rates, and the severity of E. coli UTIs. Examine if any risk factors, such as comorbidities or immunocompromised condition, are linked to a higher chance of hospitalisation for serious infections. Clinical judgement and the distribution of resources can be influenced by knowledge of the factors that indicate a serious illness [30-32].
Examine the frequency of complications and lasting consequences that follow UTIs caused by E. coli and how they affect the course of therapy. Extended hospital stays, elevated morbidity, and impaired renal function can result from complications such pyelonephritis, infection, and renal damage. Examine the risk factors that put people at risk for these issues and the effects they have on long-term health and quality of life.
Evaluate how patient compliance to treatment plans affects how well E. coli UTIs are treated. The mishandling of antibiotics, whether by incorrect dosage or early cessation, can lead to treatment failure, recurring infections, and resistance to antibiotics. Examine the effects of patient adherence techniques on treatment efficacy, including instruction for patients, streamlined dose regimens, or the implementation of reminders systems.
Analyse how different risk variables related to E. coli UTIs affect the course of treatment and its results. These risk factors might include things like sexual activity, urinary tract instrumentation, a catheterization, anatomical anomalies, age, gender, and underlying medical disorders like diabetes or urinary tract abnormalities. Personalised approaches to UTI management and optimal treatment results may be achieved by comprehending the interactions between these risk variables and treatment methods (Figure 7).
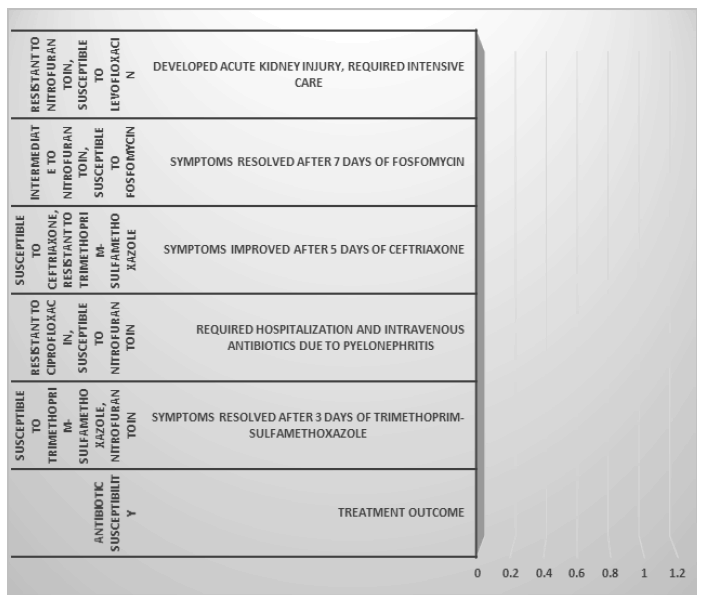
Figure 7: Treatment according to antibiotic susceptibility.
Conclusion
To sum up, this dissertation has clarified the many risk factors linked to the development of Urinary Tract Infections (UTIs) and the prevalence of E. coli. E. coli is still the most frequent cause of UTIs, according to a thorough assessment of the literature and empirical research. This emphasises the need of comprehending its epidemiology and related aspects for efficient treatment and preventive measures.
The results of this investigation highlight how important anatomical features, behavioural patterns, comorbidities, age, and gender are in determining an individual's susceptibility to UTIs caused by E. coli. Additionally, the necessity for prudent antibiotic usage and the investigation of alternative therapeutic options is highlighted by the role that antimicrobial resistance plays in complicating treatment results.
Furthermore, the discovery of new risk variables, such dietary practices and socioeconomic status, sheds light on the complex nature of E. coli UTIs and highlights the need of treating this public health issue holistically.
Subsequent study attempts have to be directed towards expanding upon the complex interplay of host, pathogen, and environmental elements in the progression and durability of urinary tract infections caused by Escherichia coli. Furthermore, initiatives should be focused on creating focused interventions and policies meant to lessen the burden of E. coli UTIs and mitigating the associated risks on both individual and population levels.
Overall, by offering a thorough understanding of the prevalence and risk factors associated with E. coli UTIs, this dissertation adds to the body of information already available on the subject, helping to guide future research paths, public health initiatives, and clinical practice.
Authors Contributions
There are two authors who are solely owner of the contents of the manuscript. All the images and tables attached here are original and prepared during the laboratory work by both the authors. The idea of the concept of the manuscript is done by rajrupa ghosh. The data collections and referencing done by shiblee sarwar. The experimental work done by both the rajrupa ghosh and shiblee sarwar.
Conflict of Interest
The authors have no conflicts of interest to declare. There are two authors who are solely owner of the contents of the manuscript and there is no financial interest to report. I certify that the submission is original work and is not under review at any other publication.
References
- Choi HJ, Jeong SH, Shin KS, Kim YA, Kim YR, Kim HS, et al. Characteristics of Escherichia coli urine isolates and risk factors for secondary bloodstream infections in patients with urinary tract infections. Microbiol Spectr. 2022;10(4):e01660-22.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Zhou Z, Zheng L, Gong Z, Li Y, Jin Y, et al. Urinary tract infections caused by uropathogenic Escherichia coli: Mechanisms of infection and treatment options. Int J Mol Sci. 2023;24(13):10537.
[Crossref] [Google Scholar] [PubMed]
- Ghosh A, Bandyopadhyay D, Koley S, Mukherjee M. Uropathogenic Escherichia coli in India—an overview on recent research advancements and trends. Appl Biochem Biotechnol. 2021;193:2267-2296.
[Crossref] [Google Scholar] [PubMed]
- Janifer J, Geethalakshmi S, Satyavani K, Viswanathan V. Prevalence of lower urinary tract infection in South Indian type 2 diabetic subjects. Indian J Nephrol. 2009;19(3):107-111.
[Crossref] [Google Scholar] [PubMed]
- Haugan MS, Hertz FB, Charbon G, Sahin B, Løbner-Olesen A, Frimodt-Møller N. Growth rate of Escherichia coli during human urinary tract infection: Implications for antibiotic effect. Antibiotics (Basel). 2019;8(3):92.
[Crossref] [Google Scholar] [PubMed]
- Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;38(8):1150-1158.
[Crossref] [Google Scholar] [PubMed]
- Masoud SS, Majigo M, Silago V, Kunambi P, Nyawale H, Moremi N, et al. Utility of dipstick urinalysis in the diagnosis of urinary tract infections among outpatients in Mwanza and Dar es Salaam regions in Tanzania. Bull Natl Res Cent. 2024;48(1):6.
- Gurung R, Adhikari S, Adhikari N, Sapkota S, Rana JC, Dhungel B, et al. Efficacy of urine dipstick test in diagnosing urinary tract infection and detection of the blaCTX-M gene among ESBL-producing Escherichia coli. Diseases. 2021;9(3):59.
[Crossref] [Google Scholar] [PubMed]
- Lepowsky E, Ghaderinezhad F, Knowlton S, Tasoglu S. based assays for urine analysis. Biomicrofluidics. 2017;11(5).
[Crossref] [Google Scholar] [PubMed]
- Fernandez E, Barlis MJ, Dematera KA, LLas G, Paeste RM, Taveso D, et al. Four-class urine microscopic recognition system through image processing using artificial neural network. J Telecommun Electron Comput Eng.(JTEC). 2018:214-218.
- Andersen H, Daae LN, Wien TN. Urine microscopy--an important diagnostic tool. Tidsskr Nor Laegefore. 2014;134(18):1765-1768.
[Crossref] [Google Scholar] [PubMed]
- Muzammil M, Adnan M, Sikandar SM, Waheed MU, Javed N, Rehman MF. Study of culture and sensitivity patterns of urinary tract infections in patients presenting with urinary symptoms in a tertiary care hospital. Cureus. 2020;12(2).
[Crossref] [Google Scholar] [PubMed]
- Frost DW, Vaisman A. The Use of Urinalysis and Urine Culture in Diagnosis: The Role of Uncertainty Tolerance. Am J Med. 2023;136(8):729-731.
[Crossref] [Google Scholar] [PubMed]
- Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615-1619.
[Crossref] [Google Scholar] [PubMed]
- Kwon JH, Fausone MK, Du H, Robicsek A, Peterson LR. Impact of laboratory-reported urine culture colony counts on the diagnosis and treatment of urinary tract infection for hospitalized patients. Am J Clin Pathol. 2012;137(5):778-784.
[Crossref] [Google Scholar] [PubMed]
- Coulthard MG. Defining urinary tract infection by bacterial colony counts: A case for 100,000 colonies/ml as the best threshold. Pediatr Nephrol. 2019;34(10):1639-1649.
[Crossref] [Google Scholar] [PubMed]
- Zhong Q, Cheng F, Liang J, Wang X, Chen Y, Fang X, et al. Profiles of volatile indole emitted by Escherichia coli based on CDI-MS. Sci Rep. 2019;9(1):13139.
[Crossref] [Google Scholar] [PubMed]
- Alqaisi AH. Some charicteristic of microbial indole extracted from pathogenic E. coli in comparable with standard one. Iraqi J Agric Sci. 2023;54(5):1193-1201.
- Yun KW, Kim HY, Park HK, Kim W, Lim IS. Virulence factors of uropathogenic Escherichia coli of urinary tract infections and asymptomatic bacteriuria in children. J Microbiol Immunol Infect. 2014;47(6):455-461.
[Crossref] [Google Scholar] [PubMed]
- Mahesh S, Carmelin DS, Muthusamy R. Bacterial Flora and Treatment Strategies in Women with Escherichia coli Urinary Tract Infections. Cureus. 2024;16(3).
[Crossref] [Google Scholar] [PubMed]
- Chang SK, Lo DY, Wei HW, Kuo HC. Antimicrobial resistance of Escherichia coli isolates from canine urinary tract infections. J Vet Med Sci. 2015;77(1):59-65.
[Crossref] [Google Scholar] [PubMed]
- Rabbee M, Begum M, Islam M, Chowdhury P, Chowdhury O, Zohora F, et al. Multidrug resistance phenotype and plasmid profiling of Escherichia coli isolates causing urinary tract infections in north east part of Bangladesh. Br Microbiol Res J. 2016;15(6):1-1.
- Yigit Y, Yazici V, Ayhan H, Gencer EG, Halhalli HC, Karakayali O, et al. The analysis of Escherichia coli resistance in urine culture and in antibiograms as requested by emergency service. Turk J Emerg Med. 2014;14(3):121-124.
[Crossref] [Google Scholar] [PubMed]
- Sabir S, Anjum AA, Ijaz T, Ali MA, Nawaz M. Isolation and antibiotic susceptibility of E. coli from urinary tract infections in a tertiary care hospital. Pak J Med Sci. 2014;30(2):389.
[Crossref] [Google Scholar] [PubMed]
- Niranjan V, Malini A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J Med Res. 2014;139(6):945-948.
[Google Scholar] [PubMed]
- Schiller H, Young C, Schulze S, Tripepi M, Pohlschroder M. A twist to the Kirby-Bauer disk diffusion susceptibility test: an accessible laboratory experiment comparing Haloferax volcanii and Escherichia coli antibiotic susceptibility to highlight the unique cell biology of archaea. J Microbiol Biol Educ. 2022;23(1):e00234-21.
[Crossref] [Google Scholar] [PubMed]
- Marrs CF, Zhang L, Foxman B. Escherichia coli mediated urinary tract infections: Are there distinct uropathogenic E. coli (UPEC) pathotypes?. FEMS Microbiol Lett. 2005;252(2):183-190.
[Crossref] [Google Scholar] [PubMed]
- Bai AD, Bonares MJ, Thrall S, Bell CM, Morris AM. Presence of urinary symptoms in bacteremic urinary tract infection: A retrospective cohort study of Escherichia coli bacteremia. BMC Infect Dis. 2020:1-0.
[Crossref] [Google Scholar] [PubMed]
- Heytens S, De Sutter A, Coorevits L, Cools P, Boelens J, Van Simaey L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect. 2017;23(9):647-652.
[Crossref] [Google Scholar] [PubMed]
- Lee DS, Lee SJ, Choe HS. Community‐Acquired Urinary Tract Infection by Escherichia coli in the Era of Antibiotic Resistance. Biomed Res Int. 2018;2018(1):7656752.
[Crossref] [Google Scholar] [PubMed]
- Naber KG, Wagenlehner F, Kresken M, Cheng WY, Catillon M, Duh MS, et al. Escherichia coli resistance, treatment patterns and clinical outcomes among females with uUTI in Germany: A retrospective physician-based chart review study. Sci Rep. 2023;13(1):12077.
[Crossref] [Google Scholar] [PubMed]
- Hashimoto M, Mao BH, Chiou CS, Huang WC, Dwija IB, Jeng SL, et al. Association between Escherichia coli with NotI-restriction resistance and urinary tract infections. J Microbiol Immunol Infect. 2022;55(4):686-694.
[Crossref] [Google Scholar] [PubMed]
Citation: Ghosh R, Sarwar S (2024). A Study on the Prevalence of E. coli in the Urinary Tract Infection and the Risk Factors Associated with It. Clin Microbiol. 13:404.
Copyright: © 2024 Ghosh R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
