Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- CiteFactor
- Ulrich's Periodicals Directory
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- Centre for Agriculture and Biosciences International (CABI)
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Scholarsteer
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Review Article - (2021) Volume 12, Issue 11
A Review on the Role of Entomopathoenic Nematodes (EPNs) in Controlling Agricultural Insect Pest
Belay Feyisa*Received: 04-Oct-2021 Published: 30-Nov-2021
Abstract
Billions of dollars are spent annually throughout the world to control and prevent Agricultural insect pest. Many bacteria, fungi, and nematodes occurring naturally in soils are known to suppress Agricultural insect pest activity. Entomopathogenic nematodes (EPNs) and their associated bacterial symbionts are highly specific in their host range and compatible with many pesticides. EPNs, also called beneficial nematodes, are commercially used to control insect pests. These nematodes offer an environmentally safe alternative to chemical insecticides, and a wide range of EPNs are effective against various Agricultural insect pests. Are soil parasites that infect different types of arthropods e.g. termites, larva of butterflies, moth, beetles and grasshopper thus affecting them in various ways. They have been utilized in classical, conservation, and augmentative biological control programs. The vast majority of applied research has focused on their potential as inundatively applied augmentative biological control agents. This is through reducing their fertility or causing sterility, delaying development and shortening longevity of the arthropods. In this review, the role of EPNs in controlling Agricultural insect pest, the current merits and limitations is summarized.
Keywords
Augmentative; Biological control; Classical; Entomopathogenic nematodes; Inundative
Introduction
Nematodes that parasitize insects, known as entomopathogenic nematodes (EPNs), have been described from 23 nematode families [1]. Of all of the nematodes studied for biological control of insects, the Steinernematidae and Heterorhabditidae have received the most attention because they possess many of the attributes of effective biological control agents of various insect pests in different habitats [2-5] and have been utilized as classical, conservational, and augmentative biological control agents [6]. Classical control involves importing and releasing the parasitoid or predator of an exotic pest that has become established in a new region. The parasitoid or predator is expected also to establish itself in its new environment, so that no further releases are necessary. Augmentative control can be divided into two sub categories: inundative release, i.e. the application of large numbers of the control organism against a pest, and seasonal inoculative release, in which the control organism is released once in a season and is expected to produce progeny that will continue to control the pest throughout the growing season. Conservation bio control refers to a whole set of measures that can be taken to favors the population buildup of indigenous natural enemies of (native) pests. The vast majority of applied research has focused on their potential as inundatively applied augmentative biological control agents [7]. Extensive research over the past three decades has demonstrated both their successes and failures for control of insect pests of crops, ornamental, and lawn and turf [8,9].
Literature Review
Objectives
The general objective of this review is to know the role of entomopathogenic nematode in controlling agricultural insect pest with the following specific objectives: to review the mass production and shelf life of entomopathogenic nematode, to review method of application and factors affecting the application of entomopathogenic nematodes (Table 1).
| Advantage | Disadvantage |
|---|---|
|
|
Table 1: Advantage and disadvantages of entomopathogenic nematodes.
Selection of entomopathogenic nematodes
Selection of an EPN for control of a particular pest insect is based on several factors that include the nematode’s host range, host finding or foraging strategy, tolerance of environmental factors and their effects on survival and efficacy (temperature, moisture, soil type, exposure to ultraviolet light, salinity and organic content of soil, means of application, agrochemicals, and others). The four most critical factors are moisture, temperature, pathogenicity for the targeted insect, and foraging strategy [10,7] Within a favorable range of temperatures, adequate moisture and a susceptible host, those EPNs with a mobile foraging strategy (cruisers and intermediate foraging strategies) could be considered for use in subterranean and certain above-ground habitats (foliar, epigeal and cryptic habitats). Those with a sit and wait foraging strategy (ambushers) will be most effective in cryptic and soil surface habitats.
The effects of environmental factors (such as temperature, moisture, aeration, and soil type [esp. texture and chemistry]) and biotic factors (species of EPN, targeted insect, age of insect, soil fauna) have been documented by numerous researchers [11,7,9]. Steinernema feltiae can be infective from 2-30°C, whereas some heterorhabditids can infect host insects from 7 to 35°C and Steinernema carpocapsae is nearly inactive at 10°C [9,12]. Currently, over 80 species of Steinernema and 20 species of Heterorhabditis have been described [13].
Biology of entomopathogenic nematode
Three unique attributes of Steinernema and Heterorhabditis nematodes make them interesting model system for application in biological control. First, they form a complex nematode bacterium mutualistic symbiosis. The bacteria are carried in the body of nematodes and released into hosts [14]. Second, they are insect pathogens with a very broad host spectrum that includes the majority of insect orders. Third, they can be cultured either in vivo or in vitro on a large scale. Even though the two groups of nematodes can infect, kill and emerge as a new generation from insects in a similar way, their life cycles are different.
The life cycle of EPN includes eggs, four juvenile stages and an adult stage. The life cycle of the entomopathogenic nematodes (EPNs) Steinernema and Heterorhabditis is subdivided into the socalled larvae stages. The infective juvenile (IJ)/ or (dauer) represents the only stage of the nematode outside of their insect host. At this stage, the nematode is a non-feeding and soil dwelling larva, encased in double cuticle with closed mouth and anus, and able to survive for long-terms in the soil. IJs of the family Heterorhabditiae use the so-called cruiser strategy to search actively in the soil for suitable insect larvae. Nematodes of the family Steinernematidae adopted the ambusher strategy, waiting passively near the soil surface for prey to cross their way. After an insect is sensed, the nematode sheds its outer cuticle to uncover mouth and anus, enters the insect through natural openings like anus, mouth and spiracles and migrates to the insect blood cavity [15]. In comparison to Steinernema, Heterorhabditis is able to penetrate directly through the thin inter segmental areas of the insect integument by using a dorsal tooth [15]. It is worth to mention that steinernematid and heterorhabditid nematodes are associated with the symbiotic bacteria Photorhabdus and Xenorhabdus [16]. The bacteria are gram negative, with facultative anaerobic rods in the family Enterobacteriaceae, and are found within the intestine of the infective juvenile (IJ) nematode [17].
The relationship between the nematode and the symbiotic bacterium is a type of symbiosis, where both benefit from the association. The nematode provides protected shelter for the symbiotic bacteria and carries the bacteria into the host. After entering the host, the nematode penetrates through the gut wall, and regurgitates symbiotic bacteria into the insect hemocoel. When the IJs enter the body cavity of a host-insect, the symbiotic bacteria are released, multiply and the host death occurs within two days [18]. The IJs complete their development and reproduce for two or three generations inside their host. The infective juveniles (IJs) are the only free-living stage and serve three main functions: dispersal, host finding, and survival under environmental detrimental conditions [9]. The bacteria break down the host tissues, and provide food sources for the nematode, which feeds and multiplies on bacterial cells and degrading host tissues. Due to the different symbiotic bacteria associated with EPN, heterorhabditid nematodes turn the host cadaver red, purple, orange, yellow, brown or sometimes green, whereas steinernematid nematodes turn the insect cadaver tan, ochre, gray or dark gray. The first stage after entering the insect is the so-called recovery phase (J3). Triggered by a unknown food signal, the nematodes exit the infective stage in a developmental step that is known as recovery and transform into the fourth stage (J4), causing a toxicogenesis by releasing an immunosuppressive factor, that inhibits antimicrobial peptides, produced by the insect (Figure 1). J4 stages nematodes develop into egg lying female or male adults in the insect cadaver and hereby run through four juvenile stages (J1 - J4) and the adult stage has up to three generations [19]. After reproduction and depletion of all nutrients, a high nematode population density triggers the nematode development into IJs again. In the case of Steinernema, IJs become colonized by bacteria via one or two founder bacterial cells. Finally, dependent on the size of the insect prey, up to several hundred thousand individuals emerge from the empty carcass. The life cycle of Heterorhabditis is similar to that of Steinernematids except for the fact that the IJs always develop into self-reproducing hermaphrodites [14]. Offspring of the first generation hermaphrodites can either develop into amphimictic adults or into automictic hermaphrodite, both can occur simultaneously. The development into amphimictic adults is induced by favorable nutritional conditions, whereas the development of hermaphrodites is induced by low concentrations of nutrient. When food is depleted, a new generation of IJs exits from the host cadaver to search new hosts [11]. The cycle from entry of IJs into a host until emergence of new IJs is dependent on temperature and varies for different species and strains. Recently, other nematode species have been shown to use pathogenic bacteria to parasitize insect hosts. Two Oscheius (Heterorhabditoides) species, O. chongmingensis and O. carolinensis, and Caenorhabditis briggsae have been identified as potential insect pathogens by baiting soil for nematodes using insect larvae as prey, a common approach used for finding EPNs [20]. All of these have been found to associate with insect pathogenic bacteria of the genus Serratia, while O. carolinensis may have additional associates [21]. O. chongmingensis and C. briggsae require their bacterial partners to cause host death, to grow and reproduce within killed insects, and emerging dauer juveniles are associated with the vectored pathogen [22]. White grubs are among the more difficult insects to control with EPNs because they have developed various morphological and behavioral barriers to infection [1]. Among white grub species that are important pests of turf in the USA, the Japanese beetle, Popillia japonica appears to be the most EPN-susceptible species [23,1] EPNs that have provided good field control of P. japonica include S. scarabaei (100%), H. bacteriophora (strain GPS11) (34–97%), H. bacteriophora (strain TF) (65–92%), and H. zealandica (strain X1) (73–98%) [23,24]. Among these, two species, H. bacteriophora and H. zealandica, are commercially available for grub control [23].
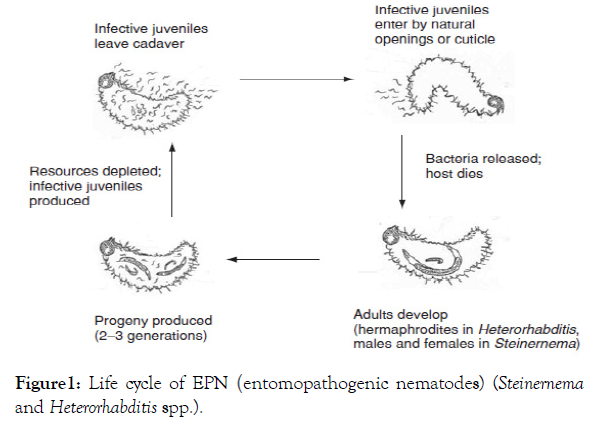
Figure 1: Life cycle of EPN (entomopathogenic nematodes) (Steinernema and Heterorhabditis spp.).
Production and formulation of entomopathogenic nematode
EPNs are currently mass produced by different methods either in vivo or in vitro [8].
In vivo mass production: In vivo production is considered the most appropriate technology for grower’s cooperatives and for developing countries, where labor is less expensive [25]. In vivo production system is based on the White trap [26] which take advantage of the IJ’s natural migration away from host cadaver upon emergence. The most common insect host used for in vivo production is the last instar of the greater wax moth Galleria melonella (L.) (Lepidoptera: Pyralidae). Producing the greater wax moth in mass has many complications, including the production of cocoons and the extreme fragility of nematode infected larvae.
The yellow mealworm, Tenebrio molitor (L.) (Coleoptera: Tenebrionidae), is an alternative host for in vivo nematode production, which does not produce cocoons and retains structural integrity, while infected by nematodes. Mealworms have the additional advantage of being produced commercially in large quantities in many countries around the world. Scientists of the U.S. Department of Agriculture, Agricultural Research Service (ARS) have developed improved systems to rear, separate, infect, and pack mealworm for production and distribution of EPNs. The structural integrity of nematode-infected mealworm cadavers has enabled the development of mechanized methods for packing, thereby reducing labor costs. Technologies developed by ARS have been implemented in a small company in the U.S. Nematodes produced in vivo using these technologies have been proven effective against the citrus weevil (Diaprepes abbreviatus) and the small hive beetle (Aethina tumida) and may be effective against other important insect pests. Methods to produce mealworms in mass do not require the use of sophisticated technology and can be implemented in less industrialized countries. Production of biological control agents can be difficult in countries where access to technology is limited. This reduces opportunities for application of biological control strategies in developing countries. Technologies developed by ARS scientists for production of nematodes using mealworms have the potential to be implemented in such countries. The most important requirement for successful and economically reasonable usage of EPNs in crop protection is large scale production at low cost within a short process time [27]. This can only be achieved under well-defined liquid culture conditions and successful management of nematode population dynamics [27]. Nowadays, EPNs are produced for commercial purposes by several companies in large liquid fermentation tanks which range from 50,000 up to 100,000 liters fermentation system [28].
In-vitro mass production : In vitro culturing of EPNs is based on introducing nematodes to a pure culture of their symbiont in a nutritive medium. A liquid medium is mixed with foam, autoclaved, and then inoculated with bacteria, followed by the nematodes. Nematodes are then harvested within 2-5 weeks [29] by placing the foam onto sieves immersed in water. Media include various ingredients including peptone, yeast extract, eggs, soy flour, and lard [30]. Nematodes can be stored and formulated in different ways including the use of polyurethane sponge, water-dispersible granules, vermiculite, alginate gels and baits.
Storage of entomopathogenic nematode
Formulated EPNs can be stored for 2 to 7 months depending on the nematode species and storage media and conditions. Unlike other microbial control agents (fungi, bacteria and virus) EPNs do not have a fully dormant resting stage and they will use their limited energy during storage. The quality of the nematode product can be determined by nematode virulence and viability assays, age and the ratio of viable to non-viable nematodes [28]. The EPNs formulated in sponges achieve a survival time of 1–3 months at 5–10°C [31] and for their release, the sponges are dipped in a bowl with water. Storage temperatures between 4 and 15°C have produced survival times of 6–12 months for steinernema spp. and 3–6 months for Heterorhabditis spp. [32] in aqueous suspension.
Method of application of entomopathogenic nematode
Nematodes have been applied successfully against soil inhabiting insects (as soil application) as well as above-ground insects (foliar spray) in cryptic habitats [33,34]. EPNs can be applied with nearly all agronomic or horticultural ground equipment’s, including pressurized sprayers, mist blowers, and electrostatic sprayers, or as aerial sprays. The application equipment used depends on the cropping system, and in each case there are a variety of handling considerations, including volume, agitation, nozzle type, pressure and recycling time, system of environmental conditions, and spray distribution pattern [35]. It is important to ensure adequate agitation during application. For small plot applications, hand held equipment or back-pack sprayers may be appropriate. When nematodes are applied to larger plots, a suitable spraying apparatus, such as a boom sprayer, should be considered. Applicators could also be using other methods, such as through micro jet irrigation systems, subsurface injection or baits [35,36]. Various formulations for entomopathogenic nematodes may be used for applying EPNs in aqueous suspension, including activated charcoal, alginate and polyacrylamide gels, clay, peat, polyurethane sponge, vermiculite, and water dispersible granules (WDG). Enhanced efficacy in EPN applications can be facilitated through improved formulation. Substantial progress has been made in recent years in developing EPN formulations, particularly for aboveground applications, such as mixing EPNs with a surfactant and polymer [37]. Improved efficacy may also be achieved by relying on leaf flooding with the addition of surfactants to increase leaf coverage [38,39].
Entomopathogen nematode-insect interactions
In nature, EPN-insect interactions involving Steinernematidae though invariably lethal to the individual insects they manage to infect, typically establish a kind of uneasy equilibrium [40]. By way of contrast, EPN-insect interactions involving the Steinernematidae that are bolstered artificially by man often produce excellent control of many important insect populations [41].
Steinernema pakistanense was found 100% mortality within one day after exposure to dose of 250 IJs. S. pakistanense found that more pathogenic than S. siamkayaii and H. indica working on termite. Odontotermis horni found that 100% mortality occurred within one day after exposure to S. pakistanense at the concentration of 100 IJs/ ml and within two days in the case of S. siamkayaii and H. indica were observed by Razia M and Sivaramakrishnan S. [42]. Shahina (2010) tested and report subterranean termite showed 96% mortality in sand assay method at the concentration of 200 IJs/ml.
S. carpocapsae caused high levels of suppression (98% efficacy in a preventative treatment) in case of the red palm weevil, Rhynchophorus ferrugineus, when applied in a chitosan formulation [43]. Steinernema riobrave caused 92.8 to 94.7% mortalities in Zeuzera pyrina larvae, when used at the concentration of 3000 IJs and 5000 IJs in the field experiment in Egypt [44]. S. siamkayai and Heterorhabditis bacteriophora caused 62.5 and 60% diamondback moth larvae mortality at dosages of 400 IJs at 60hrs whereas; S. carpocapsae caused 92.5% larval mortality [45]. The S. thermophilum caused DBM mortality of 46%, at 400 IJs/ml [46]. Application of IJs of S. carpocapsae or S. feltiae at 2.5 × 106 IJs/tree or 1-2.5 × 109/ha under optimal conditions of temperature and moisture (20-25°C, saturated humidity) can provide up to 90% reduction of overwintering larvae [41,12]. The initial introduction of S. scapterisci, mole cricket populations at release sites were reduced by up to 98%. Larval mortality of over 90% has been reported for field trials with S. riobrave when applied at 1.2 × 1010 IJs/ha [47,8,48]. S. pakistanense caused higher mortality ratio at both dose levels (57.2 and 74%) as compared to other EPN species tested on spotted bollworm E. insulana than E. vitella (55.6 and 70.8%). The mortality response of American bollworm H. armigera was 63.9 and 69% by the two doses, whereas pink bollworm P. gossypiella showed mortality rate of up to 57.6 and 71.2% by the two doses, respectively. Nyasani conducted laboratory bioassays and recorded 86.7% mortality of DBM larvae at 200 IJs/mL of S. weiseri within 72 hrs recorded 100% mortality of DBM within 48 hrs of infection, with Steinernema thermophilum. More Furthermore, mentioned that when EPN applied with the sprayable fire-gel or wood flour foam as a protecting agent for controlling the codling moth in apple tree trunks, Cydia pomonella (L.), treatments were enhanced and improved. Another novel application approach that has gained attention is delivery of EPNs in their infected host cadavers [49].
Another most stricking observation is the fact that application of EPNs in capsules, prepared from several compounds, including polysaccharide extracted from the algae, Laminaria spp. [50] are easy to apply in the field. From these capsules entomopathogenic nematodes can easily break through, and successfully infect insect pests, such as Diabrotica virgifera. In addition, these nematodefilled capsules can attract insect pests in the field if they are coated with insect food stimulant or attractants [50]. Application of cadavers may be facilitated through formulations that have been developed to protect cadavers from rupture and improve handling process [49], and development of mechanized equipment for field distribution [51]. The period of six to ten days between infection and application on soil of Galleria mellonella cadavers resulted in higher emergence of IJs and was thus recommended when using the cadaver application approach. Lately, Deol YS, et al. [52] stated that nematodes applied in host cadavers were effective and persistent when added to bags of potting media for subsequent distribution to target pest sites.
Factors that affect application of EPN in the field
Factors that affect the application of EPNs in the field include: markets, crops and target insects, formulation and shelf life, usage directions, technical support, cost and others [9,53].
Conclusion and Recommendations
The excessive use of chemical pesticides in agriculture causes serious damage to soil, air, water, flora, fauna and human beings. Therefore, it is necessary to develop environmentally friendly alternatives to control soil pests, such as entomopathogenic nematodes (EPNs). Since EPN species are host specific, they can be used selectively for the target organisms. In nature, EPN-insect interactions involving Steinernematidae though invariably lethal to the individual insects they manage to infect, typically establish a kind of uneasy equilibrium. Now a day, the use of EPNs as biopesticides against agricultural insect pests has grown rapidly in recent years. These bio-pesticides play a great role in producing organic crops and export commodities. So, researchers and higher institutions should have to give attention in producing, formulating and storing environmental safe bio-pesticides.
Acknowledgement
The author wishes to acknowledge Ambo Agricultural Research Centers and all Authors of the paper that he uses in a reference.
REFERENCES
- Klein MG, Grewal PS, Jackson TA, Koppenhofer AM. Lawn, turf and grass land pests: in (L. A. Lacey and H. K. Kaya, eds). Field manual of techniques in invertebrate pathology: Application and evaluation of pathogens for control of insects and other invertebrate pests, (2nd ed). Dordrecht: Springer. 2007;655–675.
- Lacey LA, Knight A, Huber J. Microbial control of lepidopteran pests of apple
orchards, in field manual of techniques in invertebrate pathology: Application and evaluation of pathogens for control of insects and other invertebrate pests, (eds. L.A. Lacey and H.K. Kaya), Dordrecht: Kluwer Academic Publishers, 2000;557- 576. - Batalla- Carrera L, Moton A, Garcia-del-Pino F. Efficacy of entomopathogenic nematodes against the Tomato leaf miner Tuta absoluta in laboratory and greenhouse conditions. Biocontrol. 2010;55:523-530.
- Mahmoud YA, Ebadah IMA, Hala Metwally MS, Saleh ME. Controlling of larvae, pupae and adults of the peach fruit fly, Bactrocera zonata (Saund.) (Diptera: Tephritidae) with the entomopathogenic nematode, Steinernema feltia. Egyptian J Biol Pest Cont. 2016;26(3):615 617.
- Saleh MME, Hala Metwally MS, Mahmoud YA. Potential of the entomopathogenic nematode, Heterorhabditis marelatus, isolate in controlling the peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tiphritidae). Egyptian J Biol Pest Cont. 2018;28(22):1-6.
- Bale JS, Van Lenteren JC, Bigler F. Biological control and sustainable food production. Philos Trans R Soc Lond B Biol Sci.2008;363(1492):761-776.
- Grewal PS, Ehlers RU, Shapiro-Ilan DI. Nematodes as biological control agents. Wallingford: CABI Publishing. (eds.) 2005a.
- Shapiro-Ilan DI, Gaugler R. Production technology for entomopathogenic nematodes and their bacterial symbionts. J Ind Microbiol Biotechnol. 2002;28:137-146.
- Georgis R, Koppenhofer AM, Lacey LA, Be´lair G, Duncan LW, Grewal PS, et al. Successes and failures in the use of parasitic nematodes for pest control. Biological Control. 2006;38:103–123.
- Campbell JF, Lewis EE, Stock SP, Nadler S, Kaya HK. Evolution of host search strategies in entomopathogenic nematodes. J Nematol. 2003;35:142–145.
- Shapiro-Ilan DI, Bruck DJ, Lacey LA. Principles of epizootiology and microbial control: in (Vega FE and Kaya HK, edn). Insect pathology, (2nd ed). San Diego: Academic Press. 2012;29–72.
- Lacey LA, Arthurs SP, Unruh TR, Headrick H, Jr Fritts R. Entomopathogenic nematodes for control of codling moth (Lepidoptera: Tortricidae) in apple and pear orchards: Effect of nematode species and seasonal temperatures, adjuvants, application equipment and post-application irrigation. Biological Control. 2006a;37:214–223.
- NCBI. NCBI taxonomy database. National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda MD, USA. Retrieved from May 2015, from http://www.ncbi.nlm.nih.gov/taxonomy. 2015.
- Poinar GO. Taxonomy and biology of Steinernematidae and Heterorhabditidae. In: Entomopathogenic nematodes in biological control. (Gaugler R and Kaya HK ed.). CRC Press: Boca Raton, Florida. 1990;23-61.
- Griffin CT, Boemare NE, Lewis EE. Biology and behaviours. In: (Grewal PS, Ehlers RU, Shapiro-Ilan DI, eds). Nematodes as biocontrol agents. CAB International, Wallingford. 2005;47–64.
- Jagdale GB, Kamoun S, Grewal PS. Entomopathogenic nematodes induce components of systemic resistance in plants: Biochemical and molecular evidence. Biol Control. 2009;51:102-109.
- Forst S, Clarke D. Bacteria-nematode symbiosis. In: (Gaugler R. edn). Entomopathogenic nematology. CAB International, Wallingford. 2002;pp.57–78.
- Poinar GO Jr, Grewal PS. History of entomopathogenic nematology. J Nematol. 44:153–161.
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Ann Rev Entomol. 2012;38:181-206.
- Nguyen KB, Hunt DJ. Heterorhabditdae: Species descriptions. In: (Nguyen KB, Hunt DJ (Edn)). Entomopathogenic nematodes: Systematics, phylogeny and bacterial symbionts. Nematology Monographs and Perspectives 5 (Series Editors: Hunt DJ & Perry RN). Leiden, Netherlands, Brill, 2007;pp.611-692.
- Torres-Barragan A, Suazo A, Buhler WG, Cardoza YJ. Studies on the entomopathogenicity and bacterial associates of the nematode Oscheius carolinensis. Biol Control. 2011;59:123-129.
- Ye WM, Torres-Barragan A, Cardoza YJ. Oscheius carolinensis n. sp (Nematoda: Rhabditidae): A potential entomopathogenic nematode from vermicompost. Nematology. 2010;12:121- 135.
- Grewal PS, Koppenhofer AM, Choo HY. Lawn, turfgrass, and pasture applications. In: (Grewal PS, Ehlers RU, and Shapiro-Ilan DI, (edn)). Nematodes as biocontrol agents. Wallngford: CABI Publishing. 2005b; Pp.115–146.
- Koppenhofer AM, Fuzy EM. Effect of soil type on infectivity and persistence of the entomopathogenic nematodes Steinernema scarabaei, Steinernema glaseri, Heterorhabditis zealandica, and Heterorhabditis bacteriophora. J Invertebr Pathol. 2006;92:11–22.
- Gaugler R. Production technology. In: Entomopathogenic nematology. New York, NY: CABI. 2002;289-310.
- White GF. A method for obtaining infective nematode larvae from cultures. Science. 1927;66:302-303.
- Ehlers R. Mass production of entomopathogenic nematodes for plant protection. Appl Microb Technol. 2001;56:623-633.
- Grewal PS, Koppenhofer AM, Choo HY. Lawn, turfgrass and pasture applications. In: Nematodes as biocontrolagents(eds Grewal PS, Ehlers RU and Shapiro Ilan DI). CABI Publishing, Wallingford, UK. 2005;pp.115–146.
- Bedding RA. Low cost in vitro mass production of Neoaplectana and Heterorhabditis species (Nematoda) for field control of insect pests. Nematologica.1981;27:109-114.
- Han R, Cao L, Liu X. Effects of inoculum size, temperature, and time on in vitro production of Steinernema carpocapsae. AgriotosNematologica. 1993;39:366-375.
- Grewal PS. Formulation and application technology. In: Entomopathogenic Nematology. Oxfordshire, CABI. 2002;265–287.
- Hazir S, Kaya HK, Stock SP, Keskin N. Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turk J Biol. 2003;27:181–202.
- Arthurs S, Heinz KM, Prasifka JR. An analysis of using entomopathogenic nematodes against above-ground pests. Bull Entomol Res. 2004;94:297–306.
- Shapiro-IIan DI, Gough DH, Piggott SJ, Fife JP. Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biol Control. 2006;38:124-133.
- Lara JC, Dolinski C, De Sousa FE, Daher RF. Effect of mini sprinkler irrigation system on Heterorhabditis baujardi LPP7 (Nematoda: Heterorhabditidae) infective juvenile. Scientia Agricola. 2008;65:433-437.
- Wright DJ, Peters A, Schroer S, Fife JP. Application technology. In: (Grewal PS, Ehlers RU & Shapiro-Ilan DI (Eds.)). Nematodes as Biocontrol Agents. CABI Publishing Wallingford, UK 2005; pp .91-106.
- Schroer S, Ehlers, RU. Foliar application of the entomopathogenic nematode Steinernema carpocapsae for biological control of diamondback moth larvae (Plutella xylostella). Biol Control. 2005;33:81–86.
- Williams EC, Walters KFA. Foliar application of the entomopathogenic nematode Steinernema feltiae against leaf miners on vegetables. Biocontrol Sci Technol. 2000;10:61–70.
- Head J, Lawrence AJ, Walters KFA. Efficacy of the entomopathogenic nematode, Steinernema feltiae, against Bemisia tabaci in relation to plant species. J Appl Entomol. 2004;128:543-547.
- Nguyen KB. Species of Steinernema. Entomology & Nematology Department, University of Florida. 2010.
- Unruh TR, Lacey LA. Control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae) with Steinernema carpocapsae: Effects of supplemental wetting and pupation site on infection rate. Biol Control. 2001;20:48–56.
- Razia M, Sivaramakrishnan S. Evaluation of entomopathogenic nematodes against Termites. J Entomol Zool Stud. 2016; 4(4): 324-327.
- Llacer E, Martinez De Altube MM, Jacas JA. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. BiolControl. 2009;54:559-565.
- Shamseldean MM, Hasanain SA, Rezk, MZA. Virulence of entomopathogenic nematodes against lepidopterous pests of horticultural crops in Egypt. 4th Conference on Recent Technologies in Agriculture. 2009;74-84.
- Sasnarukkit A. Efficacy of an Entomopathogenic Nematode, Steinernema siamkayai
stock, somsook and reid on controlling diamondback moth, Plutella xylostella(Linnaeus). (Ph.D. thesis), Kasetsart University. 2003. - Somvanshi VS, Ganguly S, Paul AVN. Field efficacy of the entomopathogenic nematode Steinernema thermophilum Ganguly and Singh (Rhabditida: Steinernematidae) against diamondback moth (Plutella xylostella L.) infesting cabbage. BiolControl. 2006;37:9-15.
- McCoy CW, Stuart RJ, Duncan LW, Nguyen K. Field efficacy of two commercial preparations of entomopathogenic nematodes against larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in alfisol type soil. Florida Entomol. 2002;85:537–544.
- Stuart RJ, El Borai FE, Duncan LW. From augmentation to conservation of entomopathogenic nematodes: Trophic cascades, habitat manipulation and enhanced biological control of Diaprepes abbreviatus root weevils in Florida citrus groves. J Nematol. 2008;40:73–84.
- Shapiro-Ilan DI, Morales-Ramos JA, Rojas MG, Tedders WL. Effects of a novel entomopathogenic nematode infected host formulation on cadaver integrity, nematode yield, and suppression of Diaprepes abbreviatus and Aethina tumida under controlled conditions. J Invertebr Pathol.2010b;103:103-108.
- Hiltpold I, Hibbard BE, French BW, Turlings TCJ. Capsules containing entomopathogenic nematodes as a Trojan horse approach to control the western corn rootworm. Plant and Soil. 2012;358:11–25.
- Zhu H, Grewal PS, Reding ME. Development of a desiccated cadaver delivery system to apply entomopathogenic nematodes for control of soil pests. Appl Eng Agric. 2011;27:317–324.
- Deol YS, Jagdale GB, Cañas L, Grewal PS. Delivery of entomopathogenic nematodes directly through commercial growing media via the inclusion of infected host cadavers: A novel approach. Biol Control. 201158:60–67.
- Lacey LA, Georgis R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J Nematol 2012;44:218–225.
Citation: Feyisa B (2021) A Review on the Role of Entomopathoenic Nematode (EPNs) in Controlling Agricultural Insect Pest. J Plant Pathol Microbiol 12:583.
Copyright: © 2021 Feyisa B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.