Indexed In
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- The Global Impact Factor (GIF)
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2019) Volume 10, Issue 1
A Review of the Usefulness of Non-invasive Exhaled Breath Condensate pH Analysis for Diseases Diagnosis with Elements of Meta-analysis: An Update from 2012
Mu�?¼a M, Konieczna L* and Bączek TReceived: 16-Dec-2018 Published: 31-Jan-2019, DOI: 10.35248/2153-2435.19.10.605
Abstract
Exhaled breath condensate (EBC) sample analysis is an entirely non-invasive novel sample collection method that is fast, easy to perform, and effort-appeared independent. EBC samples can be very useful in identifying the biomarkers of many diseases. This review provides an updated overview of EBC pH disturbances in different disorders as well as physiological levels among healthy individuals since 2012. Our meta-analysis addresses some of the key questions related to sample processing before pH measurement and discusses various methods of condensate standardization that can be employed prior to conducting a pH assay. Given the recent widespread interest in research into the use of EBC to identify biomarkers, it is necessary to establish a pathway leading from analytical methods for biomarker evaluation using EBC pH to clinical applications of this technology. This review fills a gap in the literature and attempts to connect theory to practical analytical approaches to analyzing EBC samples and making critical treatment-related decisions next to the patient’s bed.
Keywords
Exhaled breath condensate (EBC) samples; Noninvasive colleting method; Biomarker; Diseases; pH; Acidification
Introduction
Exhaled breath condensate (EBC) is a new type of biological sample that is sources of numerous biomarkers, which may potentially be useful in helping clinicians, diagnose various illnesses. Although respiratory disorders alter the chemical compounds in EBC, which makes it possible to detect them, these compounds can also be affected by systemic diseases. Therefore, EBC can also be used to detect both respiratory and systemic diseases. EBC sample collection is an entirely non-invasive and highly simple process that can be performed on spontaneously breathing patients, as well as on those who are being assisted by mechanical ventilators.
EBC sample analysis has gained much attention in recent years, with many studies showing its numerous possible clinical applications. Specifically, EBC sample analysis can be useful for diagnosing a number of conditions, as these conditions cause chemical changes within EBC. Some examples of such conditions include: asthma, chronic obstructive pulmonary disease (COPD), lung cancer, mechanical ventilation, idiopathic pulmonary fibrosis (IPF), cystic fibrosis, pulmonary arterial hypertension (PAH), obstructive sleep apnea (OSA), sarcoidosis, systemic lupus erythematosus (SLE), and chronic renal disease (CRD). In addition, EBC may also be useful in pharmacokinetics [1].
Since many conditions can be detected via disturbances in the pH of EBC samples, this simple test could have numerous potential clinical applications. Given this potential, we have compiled a summary of previous studies that examine EBC pH analysis in order to provide researchers with a useful tool that can assist them in introducing this analytical approach into their everyday clinical practice.
There are a few new reviews of EBC analysis [1-4] in which the authors discuss the methodological problems associated with EBC pH analysis, as well as its possible clinical applications for different compounds. Furthermore, the number of trials that have identified new biomarkers and levels of known markers in different conditions has been increasing rapidly. Indeed, it seems to be impossible to adequately detail all of these articles in one paper. Changes in concentrations of non-volatile as well as volatile compounds in EBC are properly described but we find a gap as far as pH disturbances are taken under consideration. Finally, we have elected to examine EBC acidification, as it seems simple to measure in principle, but is much harder to quantify in practice (especially if it may serve as clinical tool).
Meta-Analysis
Methods
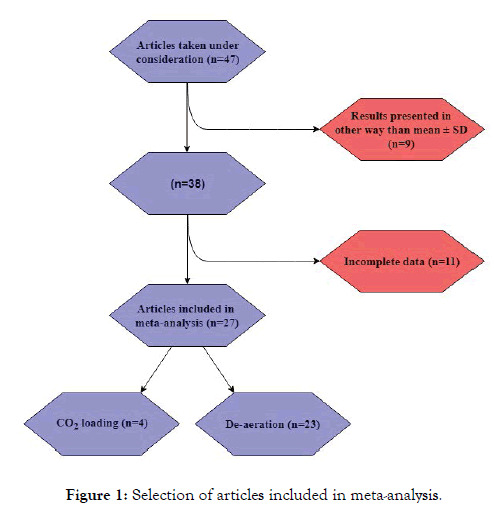
In order to estimate EBC pH values and examine the possible influence particle factors, we used the data in the below-cited articles [5-7] to perform a simple meta-analysis of the average EBC pH values in healthy controls. In total, 47 articles were taken into consideration. Studies with incomplete data (e.g. lack of de- aeration time, lack of collection device name) or results that were not presented using mean ± SD (e.g. media ± IQR) were excluded from the statistics. Once these articles had been eliminated, we were left with 27 articles (Figure 1) and a total sample of 472 individuals for analysis. We conducted our analysis using Statistica 2013 software. In order to properly strengthen data obtained from trials with greater study group we decided to introduce such parameter as number of participants. For this reason, the weights of the examples were used in all statistics. As a weight for the data number of individuals in each particular trial was set. As such, our results are weighted means and SD.
Figure 1: Selection of articles included in meta-analysis.
The analysis contains comparisons (performed with t student test) of the mean EBC pH levels between trials differing in parameters, such as: gas-standardization technique (CO2-loading vs. de-aeration with CO2-free gas); type of CO2-free gas (argon vs. nitrogen); type of collection device (R-Tube vs. EcoScreen); time of de-aeration (10- 15 minutes-which is acknowledged by many authors to be enough for equilibrium-and 20 minutes); and the correlation measured between the mean pH of the samples and the time required for the de-aeration process.
Results
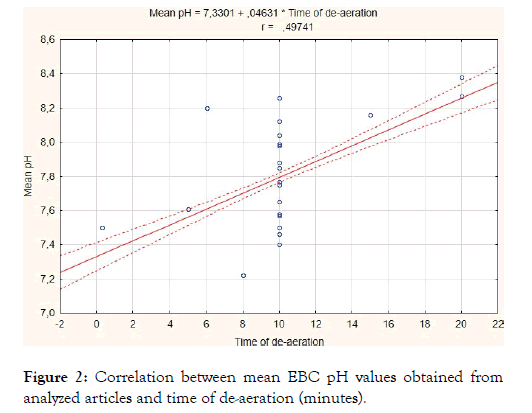
First, we compared the EBC pH values calculated after CO2 loading [8] with those obtained after de-aeration with CO2-free gas (argon or nitrogen). Since CO2 loading significantly decreased pH results (6.45 ± 0.15 vs. 7.78 ± 0.32, p<0.001, total participants number of included articles: 72 vs. 400), further analysis was performed on the results obtained after CO2-free gas standardization (n=400). The resultant calculations showed that the sample collection device and the type of gas used for standardization had no effect on average EBC pH (Table 1). However, it was found that de-aeration time and EBC ph are statistically significantly (weighted) correlated (r=0.50, p<0.05) (Figure 2). The mean pH values obtained after 20 minutes of de-aeration were significantly higher than those obtained after 10-15 minutes of gas standardization (8.34 ± 0.05 vs. 7.83 ± 0.26, p<0.001) (Table 1).
Figure 2: Correlation between mean EBC pH values obtained from analyzed articles and time of de-aeration (minutes).
Table 1: Results of meta-analysis.
Compared parameters |
N | EBC pH of healthy individuals Weighted means ± SD |
p | |
|---|---|---|---|---|
| Gas standardisation method* | CO2 loading | 72 | 6.45 ± 0.15 | <0.001 |
| De-aeration with CO2-free gas | 400 | 7.78 ± 0.32 | ||
| CO2-free gas used for de-aeration | Argon | 359 | 7.78 ± 0.32 | 0.55 |
| Nitrogen | 41 | 7.76 ± 0.34 | ||
| Collection device1 | R-tube | 104 | 7.74 ± 0.42 | 0.37 |
| EcoScreen | 157 | 7.78 ± 0.25 | ||
| De-aeration time* | 10-15 minutes | 303 | 7.83 ± 0.26 | <0.001 |
| 20 minutes | 15 | 8.34 ± 0.05 | ||
*statistically significant differences
1Two of the most common devices were compared. The rest of the authors used other devices or glass tubes with different kinds of cooling (wet ice, dry ice, liquid nitrogen).
Sample Issues
CO2 issue
Carbon dioxide represents one of the most important problems in pH analysis. The level of CO2 in EBC is highly important because it is a precursor of carbonic acid. As such, there is a critical need to reduce the impact of CO2 on EBC pH, as the acidic pH of EBC containing CO2 does not contribute to the real acidification of airways. Fortunately, the effects of CO2 can be reduced via gas standardization, which is particularly necessary for samples that are not immediately analyzed following collection. There are 2 methods of gas standardization: CO2-free gas (e.g. argon) de-aeration and CO2-loading. The main problem with CO2-free gas standardization is that such gasses, which shouldn't contain carbon dioxide, are not really CO2 free. Since the gas being used to standardize the EBC is contaminated by an unknown amount of CO2, the results of pH assays using this method tend to have poor reproducibility and may differ between laboratories [9-12]. One potential solution to this issue is by carbon dioxide loading the EBC samples and performing further statistical analysis, as this can help ensure better inter-laboratory comparison and repeatability [8]. While there are a number of CO2-free gas standardization techniques, argon-gas standardization is the most prevalent. Using this technique, the median pCO2 can be reduced from 4.08 kPa to 0.36 kPa and the median pH can be increased from 6.15 to 7.40 with 15 minutes of bubbling. EBC pH and pCO2 have a statistically significant correlation (r=-0.72, p<0.0001) in neat samples, which is eroded by the argon de-aeration process (r=0.26, p=0.11). This proves that carbon dioxide considerably influences EBC acidification. After CO2 loading and calculating EBC pH values at pCO2=5.33 kPa, the median pH was 1.3 lower than it was with argon standardization. Despite this, there is a strong positive correlation between the respective pH results following argon and CO2 standardization (r=0.88, p<0.0001) [13].
When selecting a gas-standardization technique, it is important to consider all relevant factors. There are 2 CO2-free gas standardization methods. The first method, bubble degassing, enables shorter standardization times (usually 60 seconds is enough according to Lin et al.), but it also results in higher EBC volume loss compared to surface degassing. In contrast, the second method, surface deaeraetion, requires 300 seconds in order to reach pH equilibrium. Bubble gas standardization’s shorter standardization times and its use of an open container help to reduce the risk of contamination and the degradation of unstable compounds in the EBC sample [12]. In opposition to these results, Koczulla et al. suggest that at least 20 minutes of de-aeration is required in order to stabilize EBC pH levels [14], which was confirmed by the results of our simple meta-analysis; the mean results obtained after 10-15 minutes of de-aeration (which is acknowledged by most of authors as sufficient to achieve equilibrium) are significantly lower than those obtained after 20 minutes of de-aeration (Table 1). Furthermore, it is also possible to expose the sample to atmospheric air for 2 hours. However, while this approach successfully produces CO2 equilibrium between the EBC sample and the air, it can also cause the degradation of some of the unstable compounds. This drawback notwithstanding, this method offers one great advantage: no sample volume loss [12].
The European Respiratory Society provides the following recommendations for EBC pH measurements: minimizing storage time and measuring pH before and after sample processing or performing pH measurements by calculating its value at CO2 level of 5.33 kPa after carbon dioxide loading [15] as introduced by Kullmann et al. [8].
Ammonia issue
Ammonia is volatile basic compound that is produced by the bacteria in the upper airways. When present in high concentrations, ammonia can inhibit the ability to measure acidity in a subject’s airways. This problem tends to be more relevant in oral EBC collection due to the large amounts of bacteria in the oral cavity. However, this issue may also be somewhat beneficial, as it can prevent pH assays from being overly sensitive in some cases. While low NH3 levels are often observed in populations with higher EBC acidity, this is not a necessary condition. People with normal EBC pH can have low concentrations of ammonia as well [10].
Thawing issue
Since some volatile acids can undergo sublimation during thawing, it is recommended that samples be thawed in a closed container, which should be shaken before opening [10].
Device issue
According to most authors, the type of commercially available collection device (EcoScreen vs. R-tube) does not influence EBC pH [16]. This assertion was confirmed by our meta-analysis (Table 1). PET devices do not affect exhaled breath condensate acidification, while the use of glass devices generally results in lower pH values [16]. Collection based on the exhaled CO2 signal (the collection process starts when a signal increase of 50% or more is detected) results in lower EBC pH values than are recorded with traditional collection approaches (p=0.011) [17].
Physiological Ph of EBC
The physiological pH of gas-standardized EBC in most studies is between 7.5 and 8.1. Values below 7.4 should be considered abnormal, as they are only found in 6% of population. Such values may occur due to conditions such as viral infections and proximal gastro esophageal reflux (GERD), which may be asymptomatic [10].
It is important to note that EBC pH is not affected by the following factors: age (except very elderly); gender; time of day; volume collected; duration and temperature of collection; acute albuterol/ salbutamol usage; methacholine induction of airway obstruction; and hypoventilation and hyperventilation [10,16,18]. Although gender does not influence EBC pH, it is noteworthy that pregnant women have higher pH values than non-pregnant individuals [19]. In addition, obesity does not influence EBC pH unless it is connected with obstructive sleep apnea [20,21]. Furthermore, storage conditions such as time and temperature do not change the pH of the sample [10]; indeed, EBC results remain stable even after 6 months of storage [18]. With respect to race, it has been found that African Americans tend to exhibit lower pH more often than other groups. Moreover, pH can be altered due to alcohol intake, recent smoking, and the oral ingestion of food or fluids. As such, it is recommended that patients abstain from smoking, drinking, or eating for 30 minutes before EBC collection [10,16]. Finally, since EBC pH can be reduced by the common cold [14], many authors elect to exclude patients who have been infected by this virus within the weeks leading up to the study.
As can be seen in Table 2, the range of “normal” EBC pH levels cited in the reviewed studies is very large (2.39 of difference between the highest and the lowest result). These differences are attributable to the different techniques that were used to measure EBC, as well as the de-aeration times that were used. Consequently, it is very difficult to form strong comparisons of the pH values between the control groups in the various studies.
Table 2: Articles included in meta-analysis.
| Mean EBC pH | Number of participants (weight in the statistics) | Gas standardisation method | Time of de-aeration [minutes] |
Collection device | |
|---|---|---|---|---|---|
| [55] | 6.39 | 28 | CO2-loading | - - - - - | ES |
| 6.02 | RT | ||||
| [56] | 6.63 | 20 | CO2-loading | - - - - - | ES |
| [8] | 6.54 | 12 | CO2-loading | - - - - - | ES |
| [88] | 6.19 | 12 | CO2-loading | - - - - - | RT |
| 6.45 | - - - - - | ES | |||
| 6.10 | - - - - - | AN | |||
| [68] | 7.50 | 20 | Argon de-aeration | 0.33 | O |
| [54] | 7.61 | 12 | Argon de-aeration | 5 | ES |
| [87] | 8.20 | 15 | Nitrogen de-aeration |
6 | RT |
| [50] | 7.22 | 35 | Argon de-aeration | 8 | RT |
| [26] | 7.85 | 15 | Argon de-aeration | 10 | ES |
| [31] | 7.50 | 26 | Nitrogen de-aeration |
10 | ES |
| [24] | 7.40 | 20 | Argon de-aeration | 10 | TD |
| [30] | 8.12 | 28 | Argon de-aeration | 10 | TD |
| [8] | 8.04 | 12 | Argon de-aeration | 10 | ES |
| [20] | 7.77 | 10 | Argon de-aeration | 10 | ES |
| [21] | 7.99 | 10 | Argon de-aeration | 10 | ES |
| [37] | 7.98 | 20 | Argon de-aeration | 10 | ES |
| [29] | 7.58 | 19 | Argon de-aeration | 10 | ES |
| [19] | 7.75 | 23 | Argon de-aeration | 10 | RT |
| [35] | 7.88 | 16 | Argon de-aeration | 10 | RT |
| [5] | 7.85 | 15 | Argon de-aeration | 10 | ES |
| [72] | 7.46 | 12 | Argon de-aeration | 10 | ES |
| [7] | 8.26 | 16 | Argon de-aeration | 10 | ES |
| [39] | 7.65 | 19 | Argon de-aeration | 10 | O |
| [41] | 7.57 | 10 | Argon de-aeration | 10 | O |
| [83] | 8.16 | 32 | Argon de-aeration | 15 | O |
| [14] | 8.27 | 5 | Argon de-aeration | 20 | RT |
| [6] | 8.38 | 10 | Argon de-aeration | 20 | RT |
| 8.41 | ES |
ES-EcoScreen, RT-R-tube, TD-Turbo DECCS, O-other.
EBC pH as a Biomarker of Clinical Conditions
Allergic rhinitis and atopic dermatitis
Brunetti et al. conducted a study, which showed that atopic rhinitis leads to lower EBC pH (7.48 vs. 7.78; p<0.005). In this study, the authors obtained their measurements following de-aeration with argon [22]. In a different work, De Prins et al. analyzed EBC pH in allergic populations, finding that asthma, allergic rhinitis, and nasal sIgE do not influence pH levels in EBC. However, the authors of this study do not specify which gas-standardization procedure was used, if one was used at all. This is a significant point, as their results can be unreliable if gas standardization was not performed [23].
Atopic dermatitis (AD) is another condition that can cause the acidification of exhaled breath condensate. Further results have demonstrated that populations with AD exhibit statistically significant (p<0.05) lower levels of pH: 7.44 vs. 7.78 [22], 7.00 vs. 7.40 [24], 8.02 vs. 8.11 [25]. Changes in airways pH are often precursors to respiratory symptoms. More trials are required to assess whether EBC pH can serve as a biomarker of respiratory disorders connected with allergies (such as asthma and rhinitis) [25].
Asthma
There are numerous works describing the possible role of EBC pH in the diagnostic and therapeutic processes in asthma patients. The following results suggest that EBC acidification may serve as a biomarker of the disease (asthma group vs. control): 7.42 vs. 7.85 (pediatric patients, p<0.0001) [26], 7.14 vs. 7.35 (p<0.05) [27], 7.53 vs. 7.85 (p<0.001) [28], 7.28 vs. 7.59 (p=0.04) [29], 7.87 vs. 8.12 (p=0.03) [30], 7.3 vs. 7.5 (p<0.05) [31], 6.49 vs. 6.64 (not de-aereted, p<0.05) [32,33]. However, other results do not reveal significant differences between stable asthma and control populations (p=0.06) [34], (7.54 vs. 7.75, p=0.12) [19], (7.96 vs. 7.88, p=0.23) [35], (7.94 vs. 7.90, p=0.8 [36]), [33]. Even if significant differences in EBC pH between stable asthmatic and control groups are controversial, almost all authors agree that exacerbation and poor control of the disease leads to a decrease in pH values. Moderate, severe, or badly controlled asthma is connected with lower EBC pH values than cases that are mild or well-controlled (7.36 vs. 7.49, p<0.005) [26], (7.54 vs. 7.73, p<0.05) [37], (7.33 vs. 7.23, p=0.0028) [38], (5.23 vs. 7.8) [39-41]; furthermore, there is no correlation between pH and FEV1 or FVC in adult [19,27,29] or pediatric population [26,30,32]. The acidity of the sample is normalized when the patient’s exacerbation period is properly treated [42]. In addition, EBC pH measurement can be used in conjunction with the specific inhalation challenge (SIC) to distinguish between work-exacerbated asthma (WEA) and occupational asthma (OA). A decrease in EBC pH after SIC of greater than 0.4 is connected with WEA, with a specificity of 100% and a sensitivity of 79% [43]. Conversely, an increase in EBC pH between 'at work' and 'off work' periods can help diagnose OA. Changes are greater in the SIC (+) than in the SIC (-) group (Δ mean pH: 0.59 vs. 0.17) [44]. There are no statistically significant differences in EBC acidification between GERD (+) and (-) groups in pediatric asthma populations (7.10 vs. 7.05, p=0.51) [34]. However, EBC pH cannot serve as a distinguishing factor between eosiniphilic and neutrophilic asthma (7.49 vs. 7.57, p<0.05) [37]. EBC pH correlates positively with ammonia levels in the sample and negatively with nitrite/nitrate, FeNO, and enosinophilia among patients with both stable and unstable forms of the asthma. Such correlations do not appear in healthy volunteers [29,45].
Furthermore, in a group of patients treated with ICS, those with stable asthma showed higher EBC pH than those whose asthma was unstable (7.32 vs. 7.10, p=0.04) [29]. While the treatment of exacerbated asthma can lead to an increase in EBC pH, the differences are not statistically significant (7.87 vs. 8.11, p=0.12) [30].
Changes in EBC pH influence the effects of asthma treatments. Acidosis and alkylosis of the airways lead to decreased airway blood flow response to inhaled albuterol (ΔQaw); thus, under such conditions, the effect of Β2-adrenergic agonists is worse than in the case of normal pH [45]. Unlike COPD patients, asthma patients show significant improvements in EBC pH following ICS treatments [28,40,41,46].
Asthmatic pregnant women also have lower EBC pH than healthy pregnant controls (7.65 vs. 8.02, p=0.006), and these results are significantly correlated with neonatal birthweight (r=0.49, p=0.047) [19]. Exercise-induced bronchoconstriction (EIB) episodes among asthmatic populations are associated lowered EBC pH, as asthma patients and healthy controls who do not experience EIB show no significant changes in EBC acidity [35]. Tobacco smoke exposure in populations of asthmatic children who are using ICS does not change airway acidification in comparison to an analogous group without such exposure [47].
EBC analysis can also identify biomarkers for special sub-group of asthma patients: individuals with an EBC pH of lower than 6.5 significantly differ from those with pH values higher than 6.5. Individuals with an EBC pH of less than 6.5 have lower FeNO levels (p<0.0001) and exhibit decreased provocative concentrations of methacholine, which produces a 20% decline in FEV1 (PC20) (p=0.03). Patients with very low pH levels generally show allergy symptoms in spring and winter more often than others [36,48].
COPD
Patients suffering from an exacerbation form of this disease have lower EBC pH compared to control (5.58 vs. 6.07, p<0.05) and stable COPD (5.58 vs. 5.97 p<0.05) [49] populations. Some studies have found that these differences do not persist when control and stable COPD populations are compared (5.97 vs. 6.07) [49,50]; in contrast, other studies have found statistically significant differences between these populations, thus suggesting EBC pH’s possible role as a biomarker of stable COPD [51], (p=0.0008) [52], (7.1 vs. 7.4, p<0.01) [53], (6.87 vs. 7.35, p<0.01) [27], (7.16 vs. 7.57, p<0.0001) [41], (6.97 vs. 7.61, p=0.03) [54]. Given these conflicting results, further trials are needed. In addition, ICS (inhaled corticosteroid) treatments were found to have no significant effect on EBC acidification in COPD patients [41,51,54]. The VC % of pred. and the EBC pH reveal a statistically significant negative correlation (r=-0.46, p=0.007), while other respiratory parameters (FEV1 % of pred., RV % of pred., DLco % of pred., LAV%) do not correlate with pH [27,54].
Cystic fibrosis
Cystic fibrosis (CF) is a genetic disease that mainly affects the pancreas and lungs. Antus et al. analyzed EBC pH in a CF population using CO2 bubbling gas standardization [8]. Their results showed no statistical differences between the CF group and the control group; that is, there is no difference in EBC acidity between people who do and do not have pseudomonas aeruginosa colonistaion. In addition, the authors further found that steroid treatment (ICS) did not influence pH. As their results showed, EBC pH is dependent on the condenser type (e.g. in CF patients- EcoScreen: 6.38 vs. R-tube: 5.94; p<0.05) and it is not correlated with factors such as FeNO, FVC, FEV1, and total nor differential cell counts in sputum [55].
In contrast, Carpagnano et al. discovered statistically significant differences (p<0.05) between their CF group and control group. In particular, they found that EBC pH was lower among patients whose condition was exacerbated: 7.85 (control) vs. 7.31 (stable CF) vs. 7.12 (exacerbation). These results were obtained via gas standardization with argon bubbling [26]. The differences between these trials suggest the necessity for further analyses of CF populations.
Lung cancer
Lung cancer, which is one of the most common neoplasms among adult populations, does not influence EBC pH. Additionally, a subgroup of these patients who also had COPD was also examined. The results of this study indicated that this particular neoplastic process also did not affect pH. Furthermore, no statistical differences in EBC pH were observed between patients with different stages of tumor growth [56,57]. However, two publications have provided conflicting reports on the impact of tumor histology. In the first study, Bikov et al. claimed that squamus cell carcinoma (SCC) causes lower EBC pH than adenocarcinoma (7.09 vs. 7.80; p<0.01) [57]. In contrast, the authors of the second work argue that tumor histology has no impact EBC pH [56]. Despite this, endobronchial localization of the tumor resulted in greater EBC acidity than the endobronchial-negative group (7.29 vs. 7.76; p=0.04). Moreover, gastroesophageal reflux disease (GERD) was found to also lower EBC pH, especially if left untreated. Nevertheless, EBC pH levels are not a predictive factor for survival in lung cancer populations. It should also be noted that other diseases, such as chronic heart failure, diabetes, and chronic renal failure, do not influence EBC pH [57].
Tuberculosis
The acidification of exhaled breath is a feature of pulmonary tuberculosis (TBC). As some results have revealed, TBC patients have a higher EBC pH when compared to a control group of individuals who do not have any pulmonary diseases (6.93 vs. 7.88, p<0.001). In addition, there is a negative correlation between pH and smear positivity level (r=-0.514, p<0.02), while no correlation appears between EBC acidification and radiological extent (p=0.795) [58].
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is connected with inflammation and oxidative stress brought about by EBC pH. The following results clearly demonstrate that OSA populations experience higher EBC acidity (lower pH) than a control group: 7.20 vs. 7.77 (p<0.01) [20], 7.48 vs. 7.99 (p<0.01) [21], 7.44 vs. 7.64 (p<0.01) [59]. There is a negative correlation between EBC pH and factors such as AHI (apnea-hipopnea index), TST SaO2<90%, BMI, and neck circumference [21]. These correlations suggest the presence of more advanced inflammatory processes in patients whose conditions are more severe [60]. Additionally, there are no statistically significant differences between the EBC pHs of healthy individuals and obese population without OSA [20,21]. Similarly, subgroups of OSA smokers and non-smokers exhibit similar EBC pH levels (7.43 vs. 7.45, p=0.7) [59]. Data on the impact of therapy are conflicting. Some suggest that CPAP plays a positive role [20] in the normalization of EBC pH, while others claim the opposite [61].
Chronic hypersensitivity pneumonitis
Ojanguren et al. proved that EBC pH measurements can be useful for diagnosing chronic hypersensitivity pneumonitis (HP). To this end, they had a group of patients suspected of suffering from HP perform a specific inhalation challenge (SIC). In the case of exposure to molds, those in the HP population showed a significant reduction in EBC pH in comparison to populations with other pulmonary diagnoses (e.g. nonspecific interstitial pneumonia, sarcoidosis, bronchiectasis, and pulmonary fibrosis). Statistical analysis showed that a reduction in EBC pH of 0.3 units is connected with 100% specificity, but with only 30% sensitivity. In the case of exposure to avian antigens, there were no statistically significant differences in pH changes between the HP populations and the population of patients with other diagnoses. Furthermore, the authors also suggested that EBC pH can be used to reduce the number of false negative results on SIC tests [62]. Further analysis will be necessary in order to fully assess the particular clinical implications of these results. A trial comparing the HP group with a healthy population will be necessary to determine whether EBC pH can serve as a biomarker of HP irrespectively of SIC.
Interstitial lung disease due to systemic sclerosis
Systemic sclerosis is a connective tissue disease that leads to interstitial lung disease due to the excessive production of collagen. Patients with ground glass opacities in the HRCT (radiology sign) have significantly lower EBC pH than people in control groups (7.4 vs. 8.0, p=0.02) [63]. Acidic ECB measurements can serve as a prognostic factor in identifying this disease. Castillo et al. conducted a study that demonstrated the correlation between lower EBC pH values and lower future DLCO % (diffusing capacity of the lung for carbon monoxide; 4 years follow-up, r=0.45, p=0.01). ROC curve analysis confirms that the chances of progression-free survival improve considerably above the pH=7.88 threshold, with a sensitivity of 0.71 and a specificity of 0.58 [63].
Pulmonary fibrosis
Pulmonary fibrosis (PF), which is a type of interstitial pneumonia that is connected with high collagen deposits, also leads to significant changes in EBC. Among many other biomarkers (8-iso, 3-NT, total protein, eCO, FeNO) EBC pH is also able to differentiate between PF and control populations (7.6 vs. 7.4, p=0.004). This parameter correlates with EBC concentrations of H2O2 (r=0.3, p=0.05) and 8-isoprostane (r=0.03, p=0.04), while there is no correlation with lung function factors [64]. PF is the only disease that leads to an increase in EBC pH, which makes this parameter more conditionspecific than in cases where pH is lowered. Unfortunately, the samples in the above study were not de-aerated in any way, so the obtained results may not be reliable. Moreover, the cohort only consisted of 20 patients, which is somewhat of a small sample. Therefore, it would be worthwhile to perform a study that utilized a larger number of people and that also de-aerated the obtained samples.
Gastroesophageal reflux disease
Gastroesophageal reflux disease is a condition wherein gastric acid rises into the esophagus; this can lead to inflammation and acidification of the airways, which is observed as low EBC pH levels [65], (6.34 vs. 7.22, p=0.03) [50]. Heffler et al. have demonstrated that EBC pH can be used in differencial diagnosis of chronic cough when all other causes have been excluded and GERD is suspected. Patients with a chronic cough due to GERD tend to have lower EBC pH levels than individuals who have a chronic cough, but no reflux (7.56 vs. 7.88, p=0.028) [66]. EBC pH can also serve as a biomarker of GERD among lung cancer patients (7.09 vs. 7.73, p=0.04) [57] but not among asthmatic patients [31,34,67]. In the case of chronic obstructive lung disease, there are conflicting data. One group of studies suggest that there are significant differences between GERD (+) and GERD (-) groups of patients suffering from COPD [50], while a separate group of studies have yielded contradictory results [67].
PPI treatment results in the normalization of EBC pH in patients with QUEST values no lower than 4 [31].
Inflammatory bowel disease
There are 2 main subtypes of inflammatory bowel disease (IBD): Crohn's disease (CD) and ulceritive colitis. The mean EBC pH of pediatric CD populations is significantly lower than in the control group (6.59 vs. 7.5; p<0.05). In addition, there is a significant correlation between PCDAI (Pediatric Crohn's Disease Activity Index) score and EBC pH (p=0.077). There is no correlation between acidification of EBC and ESR (p=0.89), nor between EBC and CRP (p=0.27) [68].
Mechanical ventilation
There are no correlations between EBC pH and factors such as initial C Reactive Protein (CRP), APACHE score, PaO2/FiO2, Tidal Volume, PEEP, arterial pH, PaCO2 or HCO3 ? in group of patients undergoing mechanical ventilation (MV) due to nonpulmonary disease [69]. The duration of MV before sample collection was found to influence EBC pH (reverse correlation, r=-0.636; p=0.048) [16,70], with one lung ventilation resulting in higher EBC acidification than normal MV. Furthermore, no statistically significant correlation was found between EBC acidification and total MV duration in a group of patients without pulmonary causes of respiratory distress [69]. Thus, it is critical to remember that the impact of MV on EBC pH changes during the analysis of the results. Despite this fact, EBC pH cannot serve as predictive factor of MV duration before weaning, nor can it help predict the risks of VAP (Ventilator-associated pneumonia) or mortality [69]. Nannini et al. also analyzed whether spontaneously condensing EBC in the trap of an expiratory arm could replace the need to use a cooling chamber. Their results showed that only spontaneous EBC collection and further argon de-aeration is comparable to cooling chamber condensation and further argon de-aeration. Unless these sample-collection methods are combined with de-aeration, they will show statistically significant differences [69]. This means that spontaneous collection in the trap can be considered as a replacement for the cooling chamber, but that the results can only be compared only de-aeration was conducted for both collection methods. Acute lung injury (ALI) causes the acidification of airways, which leads to lower EBC pH. There is a negative correlation between pH and inflammatory cytokine levels in EBC. Increased acidity in exhaled breath condensate can be an indication of inflammatory processes in the airways hours before clinical symptoms are observed. EBC can also serve as a biomarker of ventilator-induced lung injury [71,72] and it is possible to perform real-time EBC pH analysis on mechanically ventilated patients. This technique has been used for many diseases, including: acute asthma, cystic fibrosis, COPD, infections from respiratory syncytial virus, acute respiratory disease syndrome (ARDS), and bronchopulmonary dysplasia. Mean results higher than 7.4 are considered to represent relative lung health. In the case of patients with respiratory diseases, the mean results were approximately 4.0-6.0 [10].
Pollution
Air pollution and exposure to certain substances are important factors that can influence airway acidity, and it is important to keep this in mind when analyzing EBC pH as a biomarker for various clinical conditions. Substances that have been proven to increase the level of hydrogen ions in exhaled breath-thus, lowering the pH value-include: O3 [73], [74], SPM (suspended particulate matter) [73,75,76], black carbon [77], NO2 [76], benzene [76], ethylbenzene [76], TiO2 [78], PAH (polycyclic aromatic hydrocarbons) [79], wood dust [80], wood smoke [81], and cigarette smoke [82,83]. Furthermore, living next to a major road is also associated with lower pH values [84].
Exercise
Exercise does not seem to change EBC pH values unless the sample is de-aerated. Greenwald et al. observed a statistically significant increase in EBC pH when it was measured immediately after sample collection, but did not observe such changes when the samples were standardized [85]. Tuesta et al. performed argon bubbling during their analysis. In this experiment, subjects exercised for 10 minutes on a cycle ergometer at a stable load equal to 30% of VO2 max. EBC pH levels were measured before and 80 minutes following exercise, with an increase being observed (ΔpH=0.10, p=0.051). However, such changes did not appear when 30 and 90 minutes trials were performed [86]. In addition, there are conflicting data relating to the significant mild increase of EBC pH after exercise (measured immediately and 60 minute after exercise ends) [87,88].
Summary
EBC acidity is a good, but nonspecific, biomarker of numerous diseases. Both respiratory and systemic disorders can influence EBC pH. Therefore, it would be fruitful to conduct further research aimed at assessing utility of this parameter in each particular field, as well as to determine the level of pH analysis sensitivity for all diseases. However, the differences in the results produced by analytical and collection methods remain a huge problem. This issue should be resolved by developing new trials that are able to define physiological levels and the border of pathology of EBC pH for each method and for all relevant types of equipment. There is also a huge need for a unified pH measurement technique (especially the unification of de-aeration time and gas used for standardization), as such a method would be immensely helpful in performing largescale studies on EBC pH and comparing the results between them. In addition, in developing this unified method, it will be necessary to define pH levels that are connected with good health (reference values). Without these steps, the everyday clinical utilization of EBC pH analysis will remain impossible.
Furthermore, many problems (e.g. gas standardization, time and temperature of storage) may be eliminated if analyses are performed with blood gas analyzers immediately after sample collection. Such an approach will surely be adopted when analysis of condensate collected from patients becomes standard clinical practice.
As this review has shown, the last 6 years have seen a succession of new trials spanning right up until the most recent review of EBC pH assays [10]. The field of EBC analysis is growing at a rapid pace, and reviews such as those written by the authors of reviews [1-4] and this one can be tremendously helpful for researchers who are still developing their knowledge of EBC.
REFERENCES
- Konstantinidi EM, Lappas AS, Tzortzi AS, Behrakis PK. Exhaled breath condensate: Technical and diagnostic aspects. Sci World J. 2015;2015:435160.
- Rahimpour E, Khoubnasabjafari M, Jouyban-Gharamaleki V, Jouyban A. Non-volatile compounds in exhaled breath condensate: Review of methodological aspects. Anal Bioanal Chem. 2018;410:6411-6440.
- Beck O, Olin AC, Mirgorodskaya E. Potential of mass spectrometry in developing clinical laboratory biomarkers of nonvolatiles in exhaled breath. Clin Chem. 2016;62:84-91.
- Pereira J, Porto-Figueira P, Cavaco C, Taunk K, Rapole S. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites. 2015;5:3-55.
- Carpagnano GE, Barbaro FMP, Cagnazzo M, Di Gioia G, Giliberti T. Use of exhaled breath condensate in the study of airway inflammation after hypertonic saline solution challenge. Chest. 2005;128:3159-3166.
- Hüttmann E, Greulich T, Hattesohl A, Schmid S, Noeske S. Comparison of two devices and two breathing patterns for exhaled breath condensate sampling. PLoS ONE. 2011;6:e27467.
- Niimi A, Nguyen LT, Usmani O, Mann B, Chung KF. Reduced pH and chloride levels in exhaled breath condensate of patients with chronic cough. Thorax. 2004;59:608-612.
- Kullman T, Barta I, Lázár Z, Szill B, Barát E. Exhaled breath condensate pH standardised for CO2 partial pressure. Eur Respir J. 2007;29:496-501.
- Liang Y, Yeligar SM, Brown LA. Exhaled breath condensate: A promising source for biomarkers of lung disease. Sci World J. 2012;2012:217518.
- Davis MD, Hunt J. Exhaled breath condensate pH assays. Immunol Allergy Clin North Am. 2012;32:377-386.
- Borrill ZL, Smith JA, Naylor J, Woodcock AA, Singh D. The effect of gas standardisation on exhaled breath condensate pH. Eur Respir J. 2006;28(1):251-252.
- Lin JL, Bonnichsen MH, Thomas PS. Standardization of exhaled breath condensate: Effects of different de-aeration protocols on pH and H2O2 concentrations. J Breath Res. 2011;5(1):011001.
- Hoffmeyer F, Berresheim H, Beine A, Sucker K, Brüning T. Methodological implications in pH standardization of exhaled breath condensate. J Breath Res. 2015;9(3):036003.
- Koczulla R, Dragonieri S, Schot R, Bals R, Gauw SA. Comparison of exhaled breath condensate pH using two commercially available devices in healthy controls, asthma and COPD patients. Respir Res. 2009;10(1):78.
- Horváth I, Barnes PJ, Loukides S, Sterk PJ, Högman M. A european respiratory society technical standard: Exhaled biomarkers in lung disease. Eur Respir J. 2017;49:1600965.
- Ahmadzai H, Huang S, Hettiarachchi R, Lin JL, Thomas PS. Exhaled breath condensate: A comprehensive update. Clin Chem Lab Med. 2013;51:1343-1361.
- Goldoni M, Corradi M, Mozzoni P, Folesani G, Alinovi R. Concentration of exhaled breath condensate biomarkers after fractionated collection based on exhaled CO2 signal. J Breath Res. 2013;7(1):017101.
- Xu C, Li N, Lin X, Chen Y, Liu C. Study on the influence factors of exhaled breath condensate pH detection of asthma children. Wei Sheng Yan Jiu. 2014;43:488-491.
- Eszes N, Bikov A, Lázár Z, Bohács A, Müller V. Changes in exhaled breath condensate pH in healthy and asthmatic pregnant women. Acta Obstet Gynecol Scand. 2013;92(5):591- 597.
- Petrosyan M, Perraki E, Simoes D, Koutsourelakis I, Vagiakis E. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breathing. 2008;12:207-215.
- Carpagnano GE, Spanevello A, Sabato R, Depalo A, Turchiarelli V. Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl Res. 2008;151(1):45-50.
- Brunetti L, Francavilla R, Tesse R, Strippoli A, Polimeno L. Exhaled breath condensate pH measurement in children with asthma, allergic rhinitis and atopic dermatitis. Pediatr Allergy Immunol. 2006;17(6):422-427.
- De Prins S, Marcucci F, Sensi L, Van De Mieroop E, Nelen V. Exhaled nitric oxide and nasal tryptase are associated with wheeze, rhinitis and nasal allergy in primary school children. Biomarkers. 2014;19(6):481-487.
- Brunetti L, Francavilla R, Tesse R, Fiermonte P, Fiore FP. Exhaled breath condensate cytokines and pH in pediatric asthma and atopic dermatitis. Allergy Asthma Proc. 2008;29(5):461-467.
- Peroni DG, Bodini A, Corradi M, Coghi A, Boner AL. Markers of oxidative stress are increased in exhaled breath condensates of children with atopic dermatitis. Br J Dermatol. 2012;166(4):839-843.
- Carpagnano GE, Barnes PJ, Francis J, Wilson N, Bush A. Breath condensate pH in children with cystic fibrosis and asthma: A new noninvasive marker of airway inflammation? Chest. 2004;125:2005-2010.
- Murata K, Fujimoto K, Kitaguchi Y, Horiuchi T, Kubo K. Hydrogen peroxide content and pH of expired breath condensate from patients with asthma and COPD. COPD J Chronic Obstructive Pulm Dis. 2014;11:81-87.
- Carraro S, Folesani G, Corradi M, Zanconato S, Gaston B. Acid-base equilibrium in exhaled breath condensate of allergic asthmatic children. Allergy Eur J Allergy Clin Immunol. 2005;60:476-481.
- Tomasiak-Lozowska MM, Zietkowski Z, Przeslaw K, Tomasiak M, Skiepko R. Inflammatory markers and acid-base equilibrium in exhaled breath condensate of stable and unstable asthma patients. Int Arch Allergy Immunol. 2012;159:121-129.
- Caffarelli C, Dascola CP, Peroni D, Ricò S, Stringari G. Airway acidification in childhood asthma exacerbations. Allergy Asthma Proc. 2014;35:51-56.
- Shimizu Y, Dobashi K, Zhao JJ, Kawata T, Ono A. Proton pump inhibitor improves breath marker in moderate asthma with gastroesophageal reflux disease. Respiration. 2007;74:558-564.
- Rosias PP, Dompeling E, Dentener MA, Pennings HJ, Hendriks HJ. Childhood asthma: Exhaled markers of airway inflammation, asthma control score, and lung function tests. Pediatr Pulmonol. 2004;38:107-114.
- Aldakheel FM, Thomas PS, Bourke JE, Matheson MC, Dharmage SC. Relationships between adult asthma and oxidative stress markers and pH in exhaled breath condensate: A systematic review. Allergy Eur J Allergy Clin Immunol. 2016;71:741-757.
- Maloča Vuljanko I, Turkalj M, Nogalo B, Bulat Lokas S, Plavec D. Diagnostic value of a pattern of exhaled breath condensate biomarkers in asthmatic children. Allergol Immunopathol. 2017;45:2-10.
- Bikov A, Galffy G, Tamasi L, Bartusek D, Antus B. Exhaled breath condensate pH decreases during exercise-induced bronchoconstriction. Respirology. 2014;19:563-569.
- Liu L, Teague WG, Erzurum S, Fitzpatrick A, Mantri S. Determinants of exhaled breath condensate pH in a large population with asthma. Chest. 2011;139:328-336.
- Barbaro MP, Spanevello A, Palladino GP, Salerno FG, Lacedonia D. Exhaled matrix metalloproteinase-9 (MMP-9) in different biological phenotypes of asthma. Eur J Intern Med. 2014;25:92-96.
- Navratil M, Plavec D, Erceg D, Bulat Lokas S, Živković J. Urates in exhaled breath condensate as a biomarker of control in childhood asthma. J Asthma. 2015;52:437-446.
- Hunt JF, Fang K, Malik R, Snyder A, Malhotra N. Endogenous airway acidification: Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694-699.
- Leung TF, Li CY, Yung E, Liu EK, Lam CW. Clinical and technical factors affecting pH and other biomakers in exhaled breath condensate. Pediatr Pulmonol. 2006;41:87-94.
- Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165:1364- 1370.
- Muñoz X, Bustamante V, Lopez-Campos JL, Cruz MJ, Barreiro E. Usefulness of noninvasive methods for the study of bronchial inflammation in the control of patients with asthma. Int Arch Allergy Immunol. 2015;166:1-12.
- Snchez-Vidaurre S, Cruz MJ, Gmez-Olls S, Morell F, Muoz X. Diagnostic utility of exhaled breath condensate analysis in conjunction with specific inhalation challenge in individuals with suspected work-related asthma. Ann Allergy Asthma Immunol. 2012;108:151-156.
- Muňoz X, Velasco MI, Culebras M, Roca O, Morell F. Utility of exhaled breath condensate pH for diagnosing occupational asthma. Int Arch Allergy Immunol. 2012;159:313-320.
- Cancado JE, Mendes ES, Arana J, Horvath G, Monzon ME. Effect of airway acidosis and alkalosis on airway vascular smooth muscle responsiveness to albuterol. BMC Pharmacol Toxicol. 2015;16:9.
- Profita M, Riccobono L, Bonanno A, Chanez P, Gagliardo R. Effect of nebulized beclomethasone on airway inflammation and clinical status of children with allergic asthma and rhinitis: A randomized, double-blind, placebo-controlled study. Int Arch Allergy Immunol. 2013;161:53-64.
- De La Riva-Velasco E, Krishnan S, Dozor AJ. Relationship between exhaled nitric oxide and exposure to low-level environmental tobacco smoke in children with asthma on inhaled corticosteroids. J Asthma. 2012;49:673-678.
- Moschino L, Zanconato S, Bozzetto S, Baraldi E, Carraro S. Childhood asthma biomarkers: Present knowledge and future steps. Paediatr Respir Rev. 2015;16:205-212.
- Warwick G, Thomas PS, Yates DH. Non-invasive biomarkers in exacerbations of obstructive lung disease. Respirology. 2013;18:874-884.
- Terada K, Muro S, Sato S, Ohara T, Haruna A. Impact of gastrooesophageal reflux disease symptoms on COPD exacerbation. Thorax. 2008;63:951-955.
- MacNee W, Rennard SI, Hunt JF, Edwards LD, Miller BE. Evaluation of exhaled breath condensate pH as a biomarker for COPD. Respir Med. 2011;105:1037-1045.
- Chhabra SK, Gupta M. Exhaled breath condensate analysis in chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2012;54:27-37.
- Lee AL, Button BM, Denehy L, Roberts S, Bamford T. Exhaled breath condensate pepsin: Potential noninvasive test for gastroesophageal reflux in COPD and bronchiectasis. Respir Care. 2015;60:244-250.
- Borrill Z, Starkey C, Vestbo J, Singh D. Reproducibility of exhaled breath condensate pH in chronic obstructive pulmonary disease. Eur Respir J. 2005;25:269-274.
- Antus B, Barta I, Csiszer E, Kelemen K. Exhaled breath condensate pH in patients with cystic fibrosis. Inflamm Res. 2012;61:1141-1147.
- Horváth I, Lázár Z, Gyulai N, Kollai M, Losonczy G. Exhaled biomarkers in lung cancer. Eur Respir J. 2009;34:261-275.
- Bikov A, Lazar Z, Gyulai N, Szentkereszty M, Losonczy G. Exhaled breath condensate pH in lung cancer, the impact of clinical factors. Lung. 2015;193:957-963.
- Tasci C, Dogan D, Aydogan M, Ocal N, Ozkaya S. The relation of exhaled breath condensate "pH" with smear positivity, acute phase reactants and radiological extent of disease in smear positive pulmonary tuberculosis. Acta Med Mediterr. 2016;32:747-751.
- Antonopoulou S, Loukides S, Papatheodorou G, Roussos C, Alchanatis M. Airway inflammation in obstructive sleep apnea: Is leptin the missing link? Respir Med. 2008;102:1399-405.
- Carpagnano GE, Lacedonia D, Foschino-Barbaro MP. Noninvasive study of airways inflammation in sleep apnea patients. Sleep Med Rev. 2011;15:317-326.
- Lloberes P, Sánchez-Vidaurre S, Ferré T, Cruz MJ, Lorente J. Effect of continuous positive airway pressure and upper airway surgery on exhaled breath condensate and serum biomarkers in patients with sleep apnea. Arch Bronconeumol. 2014;50:422- 428.
- Ojanguren I, Cruz MJ, Villar A, Sanchez-Ortiz M, Morell F. Changes in PH in exhaled breath condensate after specific bronchial challenge test in patients with chronic hypersensitivity pneumonitis: A prospective study. BMC Pulm Med. 2015;15:109.
- Guillen-del Castillo A, Sánchez-Vidaurre S, Simeón-Aznar CP, Cruz MJ, Fonollosa-Pla V. Prognostic role of exhaled breath condensate pH and fraction exhaled nitric oxide in systemic sclerosis related interstitial lung disease. Arch Bronconeumol. 2017;53:120-127.
- Chow S, Thomas PS, Malouf M, Yates DH. Exhaled breath condensate (EBC) biomarkers in pulmonary fibrosis. J Breath Res. 2012;6:016004.
- Shimizu Y, Dobashi K, Nagoshi A, Kawamura O, Mori M. Assessment of airway inflammation by exhaled breath condensate and impedance due to gastroesophageal reflux disease (GERD). Inflamm Allergy Drug Targets. 2009;8:292- 296.
- Heffler E, Crimi C, Brussino L, Nicola S, Sichili S. Exhaled breath condensate pH and cysteinyl leukotriens in patients with chronic cough secondary to acid gastroesophageal reflux. J Breath Res. 2017;11:016002.
- Timms C, Thomas PS, Yates DH. Detection of gastrooesophageal reflux disease (GORD) in patients with obstructive lung disease using exhaled breath profiling. J Breath Res. 2012;6:016003.
- Huang Y, Lemberg DA, Day AS, Dixon B, Lea S. Markers of inflammation in the breath in paediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;59:505-510.
- Nannini LJ, Quintana R, Bagilet DH, Druetta M, Ramírez M. Exhaled breath condensate pH in mechanically ventilated patients. Med Intensiva. 2012;37:593-599.
- Roca O, Gómez-Ollés S, Cruz MJ, Muñoz X, Griffiths MJ. Mechanical ventilation induces changes in exhaled breath condensate of patients without lung injury. Respir Med. 2010;104:822-8.
- Roca O, Masclans JR. Exhaled breath condensate in critically ill mechanically ventilated patients. Clin Pulm Med. 2012;19:84- 89.
- Gessner C, Hammerschmidt S, Kuhn H, Seyfarth H, Sack U. Exhaled breath condensate acidification in acute lung injury. Respir Med. 2003;97:1188-1194.
- Liu L, Poon R, Chen L, Frescura A, Montuschi P. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117:668-674.
- Chen J, Zhao Q, Liu BB, Wang J, Xu HB. Airway oxidative stress and inflammation markers in chronic obstructive pulmonary diseases (COPD) patients are linked with exposure to trafficrelated air pollution: A panel study. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50:411-417.
- Cavalcante de Sá M, Nakagawa NK, Saldiva de André CD, Carvalho-Oliveira R, de Santana Carvalho T. Aerobic exercise in polluted urban environments: Effects on airway defense mechanisms in young healthy amateur runners. J Breath Res. 2016;10:046018.
- Martins PC, Valente J, Papoila AL, Caires I, Araújo-Martins J. Airways changes related to air pollution exposure in wheezing children. Eur Respir J. 2012;39:246-253.
- De Prins S, Dons E, Van Poppel M, Int Panis L, de Mieroop EV. Airway oxidative stress and inflammation markers in exhaled breath from children are linked with exposure to black carbon. Environ Int. 2014;73:440-446.
- Pelclova D, Zdimal V, Kacer P, Fenclova Z, Vlckova S. Leukotrienes in exhaled breath condensate and fractional exhaled nitric oxide in workers exposed to TiO2 nanoparticles. J Breath Res. 2016;10:036004.
- Barraza-Villarreal A, Escamilla-Nuñez MC, Schilmann A, Hernandez-Cadena L, Li Z. Lung function, airway inflammation, and polycyclic aromatic hydrocarbons exposure in mexican schoolchildren: A pilot study. J Occup Environ Med. 2014;56:415-419.
- Ćalušić AL, Varnai VM, Čavlović AO, Šegvić Klarić M, Beljo R. Respiratory health and breath condensate acidity in sawmill workers. Int Arch Occup Environ Health. 2013;86:815-825.
- Ferguson MD, Semmens EO, Dumke C, Quindry JC, Ward TJ. Measured pulmonary and systemic markers of inflammation and oxidative stress following wildland firefighter simulations. J Occup Environ Med. 2016;58:407-413.
- Kostikas K, Minas M, Nikolaou E, Papaioannou AI, Liakos P. Secondhand smoke exposure induces acutely airway acidification and oxidative stress. Respir Med. 2013;107:172- 179.
- Nicola ML, De Carvalho HB, Yoshida CT, Dos Anjos FM, Nakao M. Young "healthy" smokers have functional and infl ammatory changes in the nasal and the lower airways. Chest. 2014;145:998-1005.
- Hu ZW, Zhao YN, Cheng Y, Guo C, Wang X. Living near a major road in beijing: Association with lower lung function, airway acidification, and chronic cough. Chin Med J. 2016;129:2184-2190.
- Greenwald R, Ferdinands JM, Teague WG. Ionic determinants of exhaled breath condensate pH before and after exercise in adolescent athletes. Pediatr Pulmonol. 2009;44(8):768-777.
- Tuesta M, Alvear M, Carbonell T, García C, Guzmán-Venegas R. Effect of exercise duration on pro-oxidants and pH in exhaled breath condensate in humans. J Physiol Biochem. 2016;72:353-360.
- Riediker M, Danuser B. Exhaled breath condensate pH is increased after moderate exercise. J Aerosol Med. 2007;20:13-8.
- Czebe K, Barta I, Antus B, Valyon M, Horváth I. Influence of condensing equipment and temperature on exhaled breath condensate pH, total protein and leukotriene concentrations. Respir Med. 2008;102:720-725.
Citation: Muża M, Konieczna L, Bączek T (2019) A Review of the Usefulness of Non-invasive Exhaled Breath Condensate pH Analysis for Diseases Diagnosis with Elements of Meta-analysis: An Update from 2012. Pharm Anal Acta 10:605. doi: 10.35248/2153-2435.19.10.605.
Copyright: © 2019 Muża M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.