Indexed In
- Academic Journals Database
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Access to Global Online Research in Agriculture (AGORA)
- Electronic Journals Library
- RefSeek
- Directory of Research Journal Indexing (DRJI)
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Mini Review - (2024) Volume 2, Issue 1
A Mini-Review on the Role of Bacteriophages in Food Safety
Nosheen Amjad1, Ali Imran2, Shumaila Yousaf3, Ahmed Faraz4, Fakhar Islam1,2*, Muhammad Sadiq Naseer1 and Saleha Tahir52Department of Food Science, Government College University, Faisalabad, Pakistan
3Nuclear Institute of Agriculture and Biology, Faisalabad, Pakistan
4Department of Pharmaceutical Sciences, University of Cyberjaya, Selangor, Malaysia
5Department of Microbiology, University of Agriculture Faisalabad, Faisalabad, Pakistan
Received: 01-Aug-2023, Manuscript No. JMBT-23-22418; Editor assigned: 03-Aug-2023, Pre QC No. JMBT-23-22418 (PQ); Reviewed: 17-Aug-2023, QC No. JMBT-23-22418; Revised: 03-Jun-2024, Manuscript No. JMBT-23-22418 (R); Published: 10-Jun-2024
Abstract
Despite their serious disadvantages, which include higher upfront costs, the possibility of malfunctions due to corrosiveness, and a negative impact on the organoleptic properties of the food and possibly its nutritional importance, conventional antibacterial techniques such as pasteurization, pressure preparation, and radioactive substances are also valid as synthetic antiseptics, in fact, reduce bacterial growth in food to varying degrees. Most importantly, these cleaning techniques remove all contaminants, including various (often helpful) microorganisms found naturally in food. One potential solution to some of these issues is bacteriophage bio-control, a common and inexpensive method that uses lytic bacteriophages taken from the environment to selectively target harmful bacteria and eliminate significantly reduce their stages of feeding. It has been claimed that using bacteriophages on food is a novel way to prevent the growth of germs in vegetables. Bacteriophages are preferred because of their selectivity, security, stability, and usage. This review highlights the role of bacteriophages in food safety and their advantages in detail.
Keywords
Phages; Food safety; Corrosiveness; Antimicrobial; Green method
Introduction
Dieticians and health specialists globally promote the intake of fresh fruits and vegetables owing to their rich content of essential vitamins, minerals, and nutrients [1]. Though, fresh produce remains a significant source of foodborne illnesses, with over 400 outbreaks related to produce reported since 1990, particularly associated with tomatoes, leafy greens, and sprouted seeds [2]. Factors like open-field cultivation and handling add to the contamination of fruits and vegetables by microorganisms, leading to spoilage and food waste along the production process [3].
To address these challenges, non-chemical approaches to food safety have gained attention, especially in the context of rising organic food making and health awareness. Bacteriophages, which are bacterial viruses capable of infecting and killing specific bacterial hosts, have emerged as potential bio-regulator agents for enhancing food safety and reducing waste [4]. By using bacteriophages, foodborne illnesses can be minimized, and food spoilage can be prevented, offering a promising solution to improving food safety and sustainability.
Various studies have explored the application of bacteriophages as antibacterial agents to enhance microbiological food safety and reduce pathogenic and spoilage microorganisms in food products such as milk, poultry, cheeses, vegetables, and fresh fruits [5,6]. This review highlights the prospective application of bacteriophages in regulating microbial contamination in fresh fruits and vegetables, dairy products, and convenience foods, presenting an innovative approach to ensure food safety and minimize food waste.
Literature Review
Foodborne illnesses: The connection to contaminated food
Foodborne illness caused by food contamination is the chief contributor to disease and mortality around the world. Approximately 250 gastrointestinal diseases have been identified, with about 9.4 million occurrences of foodborne outbreaks listed annually in the U.S. alone, ensuing about 56,000 hospitalizations and 1300 fatalities [7]. The widely held of these cases are attributed to specific foodborne pathogens such as Shigella, Salmonella, Campylobacter, Listeria monocytogenes, and Escherichia coli pathotypes, along with other enteric microorganisms. The claim for fresh produce is a significant factor contributing to these incidences, often related to inadequate thermal storage and microbiologic contamination of equipment [8].
In addressing the emergence of bacterial resistance, bacteriophages, specialized viruses that target bacteria by rupturing their cell walls, are being explored as an alternative to antibiotics. Bacteriophages possess RNA or DNA genomes and can yield endolysin enzymes that split peptidoglycan, leading to cell wall lysis [9]. Furthermore, the bacteriophage genome comprises proteins known as amurins, which inhibit cell wall formation, causing cell wall rupture.
Bio-control capability of bacteriophages against dietary pathogens
Since the discovery of bacteriophages by Francis type of circuit and Walter d'Herelle a century earlier [10], researchers have demonstrated their potential in curing microbial enterococcus illnesses like cholera, correctly selected, and giardiasis, as well as a variety of acute or prolonged pathogens in fields such as cardiology, gastroenterology, neonatology, and multiple surgeries. These infectious agents have been used for various agricultural, animal, and human applications, but their application in local food production remains unexplored [11]. Contaminated food episodes associated with fresh produce have emphasized the need for concrete methods to eradicate harmful bacteria from food. However, traditional commercial sanitizers have been shown to have limitations in removing pathogens from the surfaces of fruits and vegetables [12].
To find better alternatives for ensuring bacterial exclusion on fresh produce, researchers have explored techniques such as radioactivity, consumable covering, nitrogen oxides, ultraviolet, climate-controlled storage, potassium permanganate, water, and viral proteins [13]. Phages, as operative and reasonable options for organic management, do not destroy the flavor of fresh food like conventional cleaning methods do. Investigating viral formulations for the overall bio-control capability of bacteriophages against dietary pathogens linked to bug of fruits and vegetables has been a focus. However, the unexpected outcomes have posed challenges in the application of phages for phytoremediation in the native food sector, which is attributed to inadequate treatment during viral concentration and limited knowledge of bacteriophage ecology [14]. Addressing these concerns can unlock the potential of phage-based bio-control strategies in ensuring food safety.
Bacteriophage treatment in antimicrobial resistance and infectious diseases
Bacteriophages, outnumbering bacterial cells by a factor of 10 in the environment as well as the intestines of both animal and human species, comprising a vast range of host organisms. Their genome sizes vary from 3.4 kilobases (kb) to around 500 kilobases (kb), containing numerous uncharacterized genes and proteins [15]. Phages undergo two discrete life cycles: Lysogenic and lytic. Lysogenic phages integrate their viral genome into the host's genetic material, while lytic phages kill infected host cells [16]. They exhibit improved selectivity and a restricted host range, binding to host cells through various receptors, such as proteins, sugars, and lipopolysaccharides (Figure 1) [17].
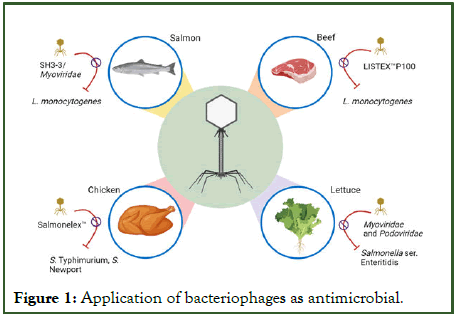
Figure 1: Application of bacteriophages as antimicrobial.
Discussion
Phages have a long history of being considered for the management of infectious diseases before the development of antibiotics. Recognized by microbiologist Felix d'Herelle in 1917, phages were primarily explored for their antibacterial activity against dangerous germ cells. Phage therapy showed promising results in controlling diseases like bacillary dysentery and cholera outbreaks [18]. However, the discovery of antibiotics in the 1930’s and 1940’s led to a decline in phage research. Challenges such as inconsistent findings, dosages, repeatability, and limited genetic information hindered further exploration.
Recent resurgence in phage research comes as an unconventional route to combat the growing threat of Antimicrobial Resistance (AMR). In Eastern Europe, phage treatment has been effectively used for around 90 years without posing health risks to patients [19]. In countries like Poland, bacteriophage treatment has proven effective against AMR diseases [20]. As current therapeutic methods falter, bacteriophage therapy offers a promising option, particularly in managing infections like Clostridioides Difficile Infection (CDI) with high fatality rates. While challenges remain, bacteriophage treatment presents a solution to combat AMR and infectious diseases.
Phage based biocontrol of food pathogens
Several experimental studies have investigated the use of phages to control pathogens in various food products and leafy green vegetables as described in Table 1 [21-24]. This indicates that bacteriophages are a promising regulator of food-borne pathogens. The table below describes the process of phage regulation and their effects on pathogenic bacteria [25-28].
| Food | Bacteria targeted | Bacteriophage used | Effects | References |
|---|---|---|---|---|
| Beef | E. coli O157 | EP75 and EP335 | Reductions of 0.8-1.1 log10 CFU/cm2 and 0.9-1.3 log10 CFU/cm2, respectively. | Witte, et al. |
| Raw meatball | E. coli O157:H7 | M8AEC16 | A reduction of 0.69-2.09 log10 CFU/g after 5 h of application. | Gencay et al. |
| Beef and lettuce | E. coli O157:H7 | EcoShield™ | Reduced the level of bacteria by ≥ 94% and 87% after 5 min contact time in meet and lettuce, respectively. | Carter, et al. |
| Beef | E. coli O157:H7 | PS5/Myoviridae | A 2.4 log10 CFU/piece after 24 h post application at 4°C, whereas a 3.5 log10 CFU/piece after 6 h post application at 24°C. | Duc, et al. |
| Chicken | S. typhimurium | PS5/Myoviridae | A 1.2 log10 CFU/piece after 24 h post application at 4°C and a 1.6 log10 CFU/piece after 6 h post application at 24°C. | Duc, et al. |
| Beef (coarse and fine ground) | S. enterica (ATCC 51741), S. Heidelberg (ATCC 8326), S. newport (ATCC 27869), and S. Enteritidis C (Se 13) | Salmonelex™ (S16 and the FO1a)/Myoviridae | Overall, a reduction of 1.6 log10 CFU/g was observed after the application of 109 phage. | Shebs, et al. |
| Ground red meat trim and poultry | S. Infantis (ATCC 51741), S. Heidelberg (ATCC 8326), S. Newport (ATCC 27869), and S. Enteritidis (SE13) | Salmonelex™ (S16 and the FO1a)/Myoviridae | Overall, phage application on trim reduced 0.8 and 1 log10 CFU/g of Salmonella in ground pork and beef, respectively, whereas a reduction of 0.9 and 1.1 log10 CFU/g occurred in ground turkey and chicken, respectively. | Yeh, et al. |
| Chicken skin | Cocktail of S. typhimurium, S. heidelberg, and S. enteritidis | SalmoFresh™ | A reduction of 0.9-1 log10 CFU/cm2 with phage only. Whereas a greater reduction of 1.6 and 1.8 log10 CFU/cm2 after 2 and 24 h. after chlorine and phage treatment. | Sukumaran, et al. |
| Chicken | S. typhimurium, S. newport, S., and Thompson | Salmonelex™ | A reduction of 0.39 log10 CFU/cm2 and 0.67 log10 CFU/cm2 after 30 min and 8 h post-inoculation, respectively. | Grant, et al. |
| Meat | L. monocytogenes | Halal-certified list-shield | A reduction of 2.3 log10 was recorded in phage treated beef samples during the storage period of 15 days. | Ishaq, et al. |
| Fresh salmon meat | L. monocytogenes | SH3-3/Myoviridae | A reduction of 2.67, 4.14, and 4.54 log10 after 24, 48, and 72 h of phage addition, respectively. | Zhou, et al. |
| Chicken | Cocktail of L. monocytogenes strains ATCC 19113, ATCC19115, and ATCC 13932 | List shield | A mean reduction of 0.56, 0.84, 0.46, and 0.10 log cycles in viable counts was observed at 0, 24, 48, and 72 h after phage treatment, respectively. | Yang, et al. |
| Cooked Turkey and roast beef | A cocktail of L. monocytogenes (serotypes; 1/2a, 1/2b, and 4b) | LISTEX™P100 | An initial reduction of 2.1 and 1.7 log10 CFU/cm2, respectively, for cooked turkey and roast beef at 4°C, while an initial reduction of 1.5 and 1.7 log10 CFU/cm2, at 10°C. | Chibeu, et al. |
| Raw chicken and pork meat | C. jejuni (NCTC 11168) and C. coli (NCTC 12668) | NCTC group II phage 12684 or CP81 | No reduction at 4°C after 7 days of inoculation. | Orquera, et al. |
| Raw and cooked beef | C. jejuni | Cj6/Myoviridae | No reduction at 5°C compared to control with low MOI. However, a 2 log10 CFU/cm2 reduction on raw and cooked meat at high host density and a high MOI of 10,000. | Bigwood, et al., |
| Chicken | C. jejuni (NCTC12662 or RM1221) | F356 and F357 | A 0.73 log10 reduction at 5°C after 24 h post-treatment. | Zampara, et al. |
| Chicken liver | C. jejuni (HPC5 and 81–176) | Phages φ3 or φ15/Myoviridae | A 0.2 to 0.7 log10 CFU/g reduction 48 h post-treatment. | Firlieyanti, et al. |
| Lettuce | Salmonella ser. enteritidis(ATCC13076) and Salmonella ser. typhimurium(ATCC14028) | BP 1369 and BP 1370/Myoviridae and Podoviridae, respectively | A reduction of >1.0 log10 CFU/cm2 after 2 h of post-treatment. | Sadekuzzaman, et al. |
| Romaine lettuce | Individual strains of STEC (EDL933; O157:H7, SN061; O26: H11, SN576; O111:NM and SN608; and O103:H2) | VE04, VE05, and VE07 | A reduction of 2.6-6 log10 CFU/cm2 after 3 days of storage at a temperature of 10°C. | Lu, et al. |
| Romaine lettuce, mung bean sprouts, and seeds | Cocktail of Salmonella strains (newport, braenderup, typhimurium, kentucky, and heidelberg | SalmoFresh™/Myoviridae | Overall reduction by spraying SalmoFresh™ onto lettuce and sprouts reduced Salmonella by 0.76 and 0.83 log10 CFU/g, respectively, whereas a reduction of 2.43 and 2.16 log10 CFU/g by immersion was observed on lettuce and sprouts, respectively. | Zhang, et al. |
| Romaine and iceberg lettuce | E. coli O157:H7 | AYO26, AXO111, AXO121, AYO145A/Myoviridae, AXO103, AKFV33/Siphoviridae, and AXO45B | A reduction of 2.6-3.2 and 1.7-2.3 log10 CFU/g for low and high contamination, respectively. | Ding, et al. |
Table 1: Application of bacteriophages in regulating food borne pathogens.
Benefits and drawbacks of using phages as antimicrobials
Phages, as antimicrobials, have both strengths and limitations as shown in Table 2 [29,30]. Their specificity in targeting diverse bacterial strains can be challenging, especially when dealing with illnesses caused by multiple strains [31-34]. While some trials have shown the safety of oral phage administration, a key concern is ensuring proper translocation of phages through the intestinal epithelium.
Studies have indicated that this translocation can be beneficial by directing the immuneresponse to innate microbial antigens and averting the development of certain inflammatory factors [35-38]. However, other research did not observe significant changes in cytokine levels after phage therapy [39]. In spite of limited data on phage treatment, it appears to have fewer side effects than conventional antibiotics and can reduce pathogenic flora in the gut [40-43].
| Benefits | Drawbacks | References |
|---|---|---|
| Phages infect only one type of bacteria, making consumer dysbiosis unlikely due to their extreme specificity. | To meet the rising demands of the food industry, phage, and phage cocktail manufacturing must be scaled significantly. | Garvey, et al.; Culot, et al. |
| Phages barely affect the organoleptic qualities of food. | To guarantee the right administration of the proper phage or phage cocktail, accurate prediction of the current pathogens is essential. | Moye, et al.; Thanki, et al. |
| A rather high level of resistance to various food preservation techniques is displayed by phages. | There is still disagreement on the stability of phages during food storage. | Garvey, et al.; Greer |
| Phages have tremendous efficacy, requiring just a tiny amount to kill germs. | Bacterial phage resistance is frequently erratic and can evolve over time. | Garvey, et al.; Salmond and Fineron |
| Against bacterial biofilms, phages have demonstrated effectiveness. | At high temperatures, phages are prone to denaturation. | Liu, et al.; Garvey, et al. |
| The term "Generally Recognised as Safe" (GRAS) is now used to describe a few goods. | The amount of chlorine in water can have an impact on the effectiveness of phages. | Vikram, et al., Zhang, et al. |
| Phages have the ability to replicate themselves, making minimal dosages necessary for effectiveness. | Pathogens can emit pro-inflammatory substances such endotoxins and peptidoglycans when they are lysed. | Tang et al. |
| Phages are appropriate for both pre and post-harvest situations due to their wide variety of applications. | Toxins carried by bacteriophages include pathogenicity islands, diphtheria toxin, cholera toxin, botulism toxin, and diphtheria toxin. | Imran, et al., Sisakhtpour, et al. |
| Phages are thought of as eco-friendly technology that is biocompatible with both people and animals. | The structure and properties of the food matrix may have an impact on how well bacteriophages work. | Garvey, et al., Vikram et al. |
| The efficacy of phages against MDR bacterial species has been established. | Bacterial endotoxins might possibly be present in crude phage lysates. | Sisakhtpour, et al., Villa, et al. |
| Bacterial endotoxins might possibly be present in crude phage lysates. | Lawpidet, et al. | |
| Possible cures and preventative measures for Clostridioides difficile (C. difficile) infections might be provided by phages. | Giau, et al. |
Table 2: Benefits and drawbacks of using bacteriophages as antimicrobials.
The regional specificity of phages allows for the selection of phages with the best infectivity contrary to target pathogens, making them potentially valuable in combating antibiotic-resistant bacteria, particularly in hospital settings [44-48]. Additionally, phages carry enzymes that can break down bacterial biofilms and extracellular polymeric materials, providing an advantage over antibiotics, which are often ineffective against biofilm-forming bacteria [49,50]. Nevertheless, further investigation is needed to completely understand and harness the potential of phages as antimicrobials [51].
Conclusion
Despite improvements in safety procedures for food, adulteration of fresh fruits and vegetables stays a significant issue. Pathogenic and deteriorative bacteria can undermine product quality and contribute to food waste. Current research in food microbiology has highlighted the effectiveness of bacteriophages in averting the growth of harmful bacteria on fresh produce. Bacteriophages are advantageous at various points of the food manufacture chain. They offer a promising auxiliary tool to combat foodborne infections, particularly concerning vulnerable populations like children, the elderly and expectant mothers. Phages are strong, definite, and selfreplicating pillagers of bacterial species, making them valuable for disease mitigation, farm-level disinfection, and food preservation. They are increasingly recognized as GRAS-Generally Recognized as Safe for use in food products and are considered organic and legitimate. Phage cocktails, in particular, have shown remarkable activity to counter Multi-Drug Resistant (MDR) species and can be combined with other safe antimicrobials, like bacteriocins, to augment effectiveness and selectivity. However, issues that require further investigation include phage resistance mechanisms, potential transmission of pathogenic genes, phage traceability in the environment, and formulation and stability challenges for therapeutic applications. Overall, Bacteriophages are essential components of ecosystems, playing a crucial character in bacterial evolution. Their use as biocontrol agents during before harvest, harvest, and after-harvest stages provides several benefits for refining food safety and sustainability, aligning with the Sustainable Development Goals (SDGs).
Acknowledgment
Authors are thankful Government College University for providing literature collection facilities.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of Interest
Authors declare that they have no conflict of interest.
Data Availability Statement
The datasets generated used and/or analyzed during the current study available from the corresponding author on reasonable request.
Consent to Participate
All the co-authors are willing to participate in this manuscript.
Consent for Publication
All authors are willing for publication of this manuscript.
References
- Fan X, Niemira BA, Doona CJ, Feeherry FE, Gravani RB. Microbial safety of fresh produce. John Wiley and Sons. 2009;41.
- Murray K, Wu F, Shi J, Jun Xue S, Warriner K. Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Qual Saf. 2017;1(4):289-301.
- Rawat S. Food spoilage: Microorganisms and their prevention. Asian J Plant Sci Res. 2015;5(4):47-56.
- O'Sullivan L, Bolton D, McAuliffe O, Coffey A. Bacteriophages in food applications: From foe to friend. Annu Rev Food Sci Technol. 2019;10:151-172.
[Crossref] [Google Scholar] [PubMed]
- Greer GG. Bacteriophage control of foodborne bacteria. J Food Prot. 2005;68(5):1102-1111.
[Crossref] [Google Scholar] [PubMed]
- Islam F, Saeed F, Afzaal M, Ahmad A, Hussain M, Khalid MA, et al. Applications of green technologies based approaches for food safety enhancement: A comprehensive review. Food Sci Nutr. 2022;10:2855-2867.
[Crossref] [Google Scholar] [PubMed]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis. 2011;17(1):7-15.
- Zaczek M, Weber-Dabrowska B, Gorski A. Phages in the global fruit and vegetable industry. J Appl Microbiol. 2015;118(3):537-556.
[Crossref] [Google Scholar] [PubMed]
- Woznica WM, Bigos J, Lobocka MB. Lysis of bacterial cells in the process of bacteriophage release-canonical and newly discovered mechanisms. Postepy Hig Med Dosw (Online). 2015;69:114-126.
[Google Scholar] [PubMed]
- Wittebole X, de Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5(1):226-235.
[Crossref] [Google Scholar] [PubMed]
- Sillankorva SM, Oliveira H, Azeredo J. Bacteriophages and their role in food safety. Int J Microbiol. 2012:863945.
[Crossref] [Google Scholar] [PubMed]
- Bhardwaj N, Bhardwaj SK, Deep A, Dahiya S, Kapoor S. Lytic bacteriophages as biocontrol agents of foodborne pathogens. Asian J Anim Vet Adv. 2015;10(11):708-723.
- Mahajan PV, Caleb OJ, Singh Z, Watkins CB, Geyer M. Postharvest treatments of fresh produce. Philosophical Transactions. Proc Math Phys Eng. 2014; 372(2017):20130309.
[Crossref] [Google Scholar] [PubMed]
- McCallin S, Sarker SA, Barretto C, Sultana S, Berger B, Huq S, et al. Safety analysis of a Russian phage cocktail: From MetaGenomic analysis to oral application in healthy human subjects. Virol. 2013;443(2):187-196.
[Crossref] [Google Scholar] [PubMed]
- Romero-Calle D, Benevides RG, Goes-Neto A, Billington CJA. Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics (Basel). 2019;8(3):138.
[Crossref] [Google Scholar] [PubMed]
- Garvey M. Bacteriophages and the one health approach to combat multidrug resistance: Is this the way? Antibiotics. 2020;9(7):414.
[Crossref] [Google Scholar] [PubMed]
- Batinovic S, Wassef F, Knowler SA, Rice DT, Stanton CR, Rose J, et al. Bacteriophages in natural and artificial environments. Pathogens. 2019;8(3):100.
[Crossref] [Google Scholar] [PubMed]
- Braga LP, Spor A, Kot W, Breuil MC, Hansen LH, Setubal JC, et al. Impact of phages on soil bacterial communities and nitrogen availability under different assembly scenarios. Microbiome. 2020:8(1):1-14.
[Crossref] [Google Scholar] [PubMed]
- Tang SS, Biswas SK, Tan WS, Saha AK, Leo BF. Efficacy and potential of phage therapy against multidrug resistant Shigella spp. Peer J. 2019;7:e6225.
[Crossref] [Google Scholar] [PubMed]
- Selle K, Fletcher JR, Tuson H, Schmitt DS, McMillan L, Vridhambal GS, et al. In vivo targeting of Clostridioides difficile using phage-delivered CRISPR-Cas3 antimicrobials. MBio. 2020;11(2):e00019-20.
[Crossref] [Google Scholar] [PubMed]
- Witte S, Huijboom L, Klamert S, van de Straat L, Hagens S, Fieseler L, et al. Application of bacteriophages EP75 and EP335 efficiently reduces viable cell counts of Escherichia coli O157 on beef and vegetables. Food Microbiol. 2022;104:103978.
[Crossref] [Google Scholar] [PubMed]
- Gencay YE, Ayaz ND, Copuroglu G, Erol I. Biocontrol of shiga toxigenic Escherichia coli O157: H7 in Turkish raw meatball by bacteriophage. J Food Saf. 2016;36(1):120-131.
- Carter CD, Parks A, Abuladze T, Li M, Woolston J, Magnone J, et al. Bacteriophage cocktail significantly reduces Escherichia coli O157: H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage. 2012;2(3):178-185.
[Crossref] [Google Scholar] [PubMed]
- Duc HM, Son HM, Yi HPS, Sato J, Ngan PH, Masuda Y, et al. Isolation, characterization and application of a polyvalent phage capable of controlling Salmonella and Escherichia coli O157: H7 in different food matrices. Food Res Int. 2020;131:108977.
[Crossref] [Google Scholar] [PubMed]
- Maurine ELS, Giotto FM, Laidler ST, de Mello AS. Effects of bacteriophages and peroxyacetic acid applications on beef contaminated with Salmonella during different grinding stages. Meat Sci. 2021;173:108407.
[Crossref] [Google Scholar] [PubMed]
- Yeh Y, Purushothaman P, Gupta N, Ragnone M, Verma SC, de Mello AS. Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products. Meat Sci. 2017;127:30-34.
[Crossref] [Google Scholar] [PubMed]
- Sukumaran AT, Nannapaneni R, Kiess A, Sharma CS. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int J Food Microbiol. 2015;207:8-15.
[Crossref] [Google Scholar] [PubMed]
- Ishaq A, Ebner PD, Syed QA, ur Rahman HU. Employing list-shield bacteriophage as a bio-control intervention for Listeria monocytogenes from raw beef surface and maintain meat quality during refrigeration storage. Lwt. 2020;132:109784.
- Zhou C, Zhu M, Wang Y, Yang Z, Ye M, Wu L, et al. Broad host range phage vB-LmoM-SH3-3 reduces the risk of Listeria contamination in two types of ready to eat food. Food Control. 2020;108:106830.
- Yang S, Sadekuzzaman M, Ha SD. Reduction of Listeria monocytogenes on chicken breasts by combined treatment with UV-C light and bacteriophage ListShield. Lwt. 2017;86:193-200.
- Chibeu A, Agius L, Gao A, Sabour PM, Kropinski AM, Balamurugan S. Efficacy of bacteriophage LISTEX™ P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int J Food Microbiol. 2013;167(2):208-214.
[Crossref] [Google Scholar] [PubMed]
- Orquera S, Golz G, Hertwig S, Hammerl J, Sparborth D, Joldic A, et al. Control of Campylobacter spp. and Yersinia enterocolitica by virulent bacteriophages. J Gene Med: Int J Biomed Res. 2012;6:273.
[Crossref] [Google Scholar] [PubMed]
- Bigwood T, Hudson JA, Billington C, Carey Smith GV, Heinemann JA. Phage inactivation of foodborne pathogens on cooked and raw meat. Food Microbiol. 2008;25(2):400-406.
[Crossref] [Google Scholar] [PubMed]
- Zampara A, Sorensen MCH, Gravesen AE, Brondsted L. Significance of phage-host interactions for biocontrol of Campylobacter jejuni in food. Food Control. 2017;73:1169-1175.
- Firlieyanti AS, Connerton PL, Connerton IF. Campylobacters and their bacteriophages from chicken liver: The prospect for phage biocontrol. Int J Food Microbiol. 2016;237:121-127.
[Crossref] [Google Scholar] [PubMed]
- Sadekuzzaman M, Mizan MFR, Yang S, Kim HS, Ha SD. Application of bacteriophages for the inactivation of Salmonella spp. in biofilms. J Food Sci Technol. 2018;24(5):424-433.
[Crossref] [Google Scholar] [PubMed]
- Lu YT, Ma Y, Wong CW, Wang S. Characterization and application of bacteriophages for the biocontrol of Shiga-toxin producing Escherichia coli in Romaine lettuce. Food Control. 2022;140:109109.
- Zhang X, Niu YD, Nan Y, Stanford K, Holley R, McAllister T, et al. SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int J Food Microbiol. 2019;305:108250.
[Crossref] [Google Scholar] [PubMed]
- Ding Y, Nan Y, Qiu Y, Niu D, Stanford K, Holley R, et al. Use of a phage cocktail to reduce the numbers of seven Escherichia coli strains belonging to different STEC serogroups applied to fresh produce and seeds. J Food Saf. 2023:e13044.
- Culot A, Grosset N, Gautier M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture. 2019; 513:734423.
- Moye ZD, Woolston J, Sulakvelidze A. Bacteriophage applications for food production and processing. Viruses. 2018;10(4):205.
[Crossref] [Google Scholar] [PubMed]
- Thanki AM, Hooton S, Gigante AM, Atterbury RJ, Clokie MR. Potential roles for bacteriophages in reducing Salmonella from poultry and swine. In Salmonella spp.-a global challenge. IntechOpen. 2021.
- Mary G. Bacteriophages and food production: Biocontrol and bio-preservation options for food safety. Antibiotics. 2022;10:1324.
[Crossref] [Google Scholar] [PubMed]
- Salmond GP, Fineran PC. A century of the phage: Past, present and future. Nat Rev Microbiol. 2015;13(12):777-786.
[Crossref] [Google Scholar] [PubMed]
- Liu S, Lu H, Zhang S, Shi Y, Chen Q. Phages against pathogenic bacterial biofilms and biofilm-based infections: A review. Pharmaceutics. 2022;14(2):427.
[Crossref] [Google Scholar] [PubMed]
- Vikram A, Woolston J, Sulakvelidze A. Phage biocontrol applications in food production and processing. Curr Issues Mol Biol. 2021;40(1):267-302.
[Crossref] [Google Scholar] [PubMed]
- Imran A, Shehzadi U, Islam F, Afzaal M, Ali R, Ali YA, et al. Bacteriophages and food safety: An updated overview. Food Sci Nutr. 2023;11:3621-3630.
- Sisakhtpour B, Mirzaei A, Karbasizadeh V, Hosseini N, Shabani M, Moghim S. The characteristic and potential therapeutic effect of isolated multidrug-resistant Acinetobacter baumannii lytic phage. Ann Clin Microbiol Antimicrob. 2022;21(1):1-11.
[Crossref] [Google Scholar] [PubMed]
- Villa TG, Siota LF, Rama JR, Sanchez-Perez A, Vinas M. Horizontal gene transfer between bacteriophages and bacteria: Antibiotic resistances and toxin production. Horizontal gene transfer: Breaking Borders Between Living Kingdoms. 2019;97-142.
- Lawpidet P, Tengjaroenkul B, Saksangawong C, Sukon P. Global prevalence of vancomycin-resistant Enterococci in food of animal origin: A meta-analysis. Foodborne Pathog Dis. 2021;18(6):405-412.
[Crossref] [Google Scholar] [PubMed]
- Giau VV, Lee H, An SSA, Hulme J. Recent advances in the treatment of C. difficile using biotherapeutic agents. Infect Drug Resist. 2019:1597-1615.
[Crossref] [Google Scholar] [PubMed]
Citation: Amjad N, Imran A, Yousaf S, Faraz A, Islam F, Naseer MS, et al. (2024) A Mini-Review on the Role of Bacteriophages in Food Safety. J Microb Biochem Technol. 16:616.
Copyright: �?�© 2024 Amjad N, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

