Indexed In
- Google Scholar
Useful Links
Share This Page
Journal Flyer

Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2023) Volume 7, Issue 3
A Comparison of Locoregional versus General Anesthesia in Patients Undergoing Carotid Endarterectomy: A Retrospective Single-Center Study
Reda El Bayoumy1*, Marc Cesarini1, Mathieu Besnard2 and Arnauld Casanova22Department of Vascular Surgery, Ajaccio Teaching Hospital, Ajaccio, France
Received: 02-Aug-2023, Manuscript No. JSA-23-22438; Editor assigned: 04-Aug-2023, Pre QC No. JSA-23-22438(PQ); Reviewed: 18-Aug-2023, QC No. JSA-23-22438; Revised: 25-Aug-2023, Manuscript No. JSA-23-22438(R); Published: 01-Sep-2023, DOI: 10.35248/2684-1606.23.7.215
Abstract
Objective: Carotid Endarterectomy (CEA) reduces the risk of stroke in patients with asymptomatic and symptomatic extracranial carotid artery stenosis. Modern medical management of extracranial carotid artery stenosis has proven its efficacy and safety; therefore, a low perioperative risk in both anesthesia and surgery is paramount. Outcomes may depend on whether Locoregional Anesthesia (LA) or General Anesthesia (GA) is used. The optimal anesthetic for CEA is controversial. To determine whether the anesthetic method correlated with the outcome of the operation, a retrospective review of 2000 consecutive carotid operations performed over a 10-year period was performed. The aim of our study was to assess the perioperative risks of CEA under locoregional anesthesia compared to those under general anesthesia.
The primary endpoint was the clinical neurological outcome.
The secondary endpoint was the mortality rate.
Design: Retrospective analytical study and prospective clinical data bank.
Patients and methods: The medical records of 2000 consecutive patients who underwent carotid endarterectomy at our institution between June 2013 and June 2023 were prospectively collected and retrospectively reviewed. Operations performed with patients under locoregional anesthesia were compared with those performed with patients under general anesthesia with respect to preoperative risk factors and perioperative complications.
Patients were divided into two groups according to intraoperative anesthetics; locoregional group: 1000 patients versus general anesthetic group: 1000 patients.
Ethical approval was obtained from relevant authorities. The requirement for patient consent was waived owing to the retrospective design of this study.
Inclusion criteria: Patients with a BMI<35 requiring extracranial carotid endarterectomy, which is considered suitable for either locoregional or general anesthesia. All patients with either symptomatic or asymptomatic extracranial carotid artery stenosis for whom surgery is advised were eligible. There were no upper age limits. Patients following thrombolysis were included. None of the patients underwent mechanical thrombectomy before surgery.
CEA was only performed by a consultant vascular surgeon and anesthetist.
The characteristics of the study groups were strictly standardized, including the exact indications for surgery, diagnostic methods, anesthetic techniques, surgical techniques (indications for and the use of intraluminal shunts, heparin dose, and patching), intraoperative monitoring, postoperative assessment, and antiplatelet therapy. Strict guidelines for anesthetic and surgical management were applied throughout the study.
The following three parameters were measured:
• Incidence of early and late perioperative strokes.
• Median length of hospital stay.
• Patient Satisfaction Index (PSI).
Confidentiality: All data obtained in this trial is kept and handled in a confidential manner in accordance with applicable laws and regulations.
Results: Perioperative stroke was more common in the GA group (3.5% vs. 0.5%; P<0.001) (Relative risk: Odds Ratio (OR), 1.4; 95% Confidence Interval (CI), 1.214–1.741). Combined death and stroke rates were none in the LA group compared to 0.6% in the GA group (P<0.001). Postoperative episodes of hypertension were more common in the LA group (72.6% vs. 46.4%; P<0.001). Hematomas requiring surgery were more common in the GA group (8.2% vs. 2.1%, P<0.001). The mortality rate was none in the LA group versus 1% in GA group (P<0.001).
Conclusion: CEA can be performed safely and efficiently under locoregional anesthesia. It improves surgical outcomes and leads to better neurological outcomes than general anesthesia.
Risk factor analysis revealed specific risk groups: Men more than women and elderly patient’s more than young patients. Asymptomatic extracranial carotid artery stenosis patients had better outcomes than post-stroke patients.
In a retrospective review of a large series of extracranial carotid operations, locoregional anesthesia was shown to be applicable to the vast majority of patients with good clinical outcomes. The versatility and safety of the locoregional anesthetic technique are sufficient for vascular anesthetists and surgeons to include it in the armamentarium of their medical skills.
Keywords
Carotid endarterectomy; Locoregional anesthesia; Cervical plexus block; Stroke; Transient ischemic attacks; Carotid cross-clamping; Surgical intraluminal shunt; Ischemia-reperfusion injury syndrome; Cerebral oximeter monitoring; Near-infrared spectroscopy
Abbreviations
CEA: Carotid Endarterectomy; CAS: Carotid Artery Stenting; LA: Locoregional Anesthesia; CPB: Cervical Plexus Block; GA: General Anesthesia; TIA: Transient Ischemic Attack; INVOS: Cerebral Oximeter Monitoring; L/min: Liter Per Minute; μg: Microgram; Kg: Kilogram; iu: International Unit; mmHg: Millimeter Mercury; mm: Millimeter; BP: Blood Pressure; MI: Myocardial Infarction; CAD: Coronary Artery Disease; AF: Atrial Fibrillation; COPD: Chronic Obstructive Pulmonary Disease; CKD: Chronic Kidney Disease; PVD: Polyvascular Disease; HDU: High Dependency Unit; vs: Versus; IV: Intravenous; NIHSS: National Institutes of Health Stroke Scale; OR: Odds Ratio; BMI: Body Mass Index; CTA: Computed Tomography Angiography; MRA: Magnetic Resonance Imaging Angiography; ASA: American Association of Anesthesiologists; BMI: Body Mass Index.
Introduction
Carotid Endarterectomy (CEA) reduces stroke risk among asymptomatic patients with extracranial carotid artery stenosis and equally reduces further stroke following a Transient Ischemic Attack (TIA) or symptomatic stroke [1] (Figure 1). Modern medical management of extracranial carotid artery stenosis has proven its efficacy and safety; therefore, a low perioperative risk in both anesthesia and surgery is paramount [2]. Outcomes may depend on whether Locoregional Anesthesia (LA) or General Anesthesia (GA) is used [2]. The optimal anesthetic for use during CEA remains controversial [3]. Anesthesia for CEA, general or locoregional, has been an issue of debate in the literature since the first Cochrane review in 1991 [4,5]. The most common technique used to anesthetize patients scheduled for CEA is general anesthesia [3]. Although CEA was often performed under LA in the 1960s [5], most surgeons prefer to operate on fully asleep and relaxed patients [5]. Reports from centers of excellence as well as a recent large meta-analysis GALA study showed no difference in patient outcome-incidence of stroke and 30-day-mortality post-surgery [5]. Nevertheless, increasing evidence favors locoregional anesthesia as an independent factor for reduced morbidity after CEA [3]. Improved outcomes of CEA performed under locoregional anesthesia are changing both surgeons and anesthetists’ attitudes [6]. Specific advantages of GA over LA include tight arterial carbon dioxide control, cerebral protection afforded by volatile anesthetics, and both surgeon and anesthetist preference for general anesthesia because of a secure airway and peace of mind [3]. However, several benefits of locoregional over general anesthesia have been suggested: “gold standard” cerebral function monitoring, intact cerebral autoregulation, reduced cardiac- and respiratory- related morbidity, lower intraluminal shunt insertion rate, shorter hospital stay, and lower cost [3,6]. Advocates of locoregional anesthesia suggest that it may reduce the incidence of perioperative complications, in addition to reducing operative time and hospital costs [3,6]. Locoregional anesthesia used during carotid surgery has been advocated as a method that allows accurate intraoperative evaluation of the patient’s neurologic status while reducing both cardiac morbidity and interference with the regulatory mechanisms of blood pressure control [3,6,7,8]. The largest available study on the subject, the GALA trial, has not shown any difference in patient outcomes–incidence of stroke and 30-day-mortality post- surgery [5,9]. However, increasing evidence supports locoregional anesthesia as an independent factor for reduced morbidity after CEA [3,6]. The advantages and disadvantages of locoregional versus general anesthesia for CEA have been well established [3,6]. Cervical Plexus Blocks (CPBs) are safe and effective anesthetic techniques; however, they may also have adverse effects [3,6]. Optimal cerebral function monitoring remains a problem that needs to be resolved [7]. Cerebral oximetry (INVOS) may be a reliable tool for predicting neurological impairment (Figure 2) [10]. Following the appropriate anesthetic, modality necessitates thorough preoperative consultation among the patient, surgeon, and anesthetist. The anesthetic plan should be made on an individual basis, taking into consideration the patient’s comorbidities and wish [6].
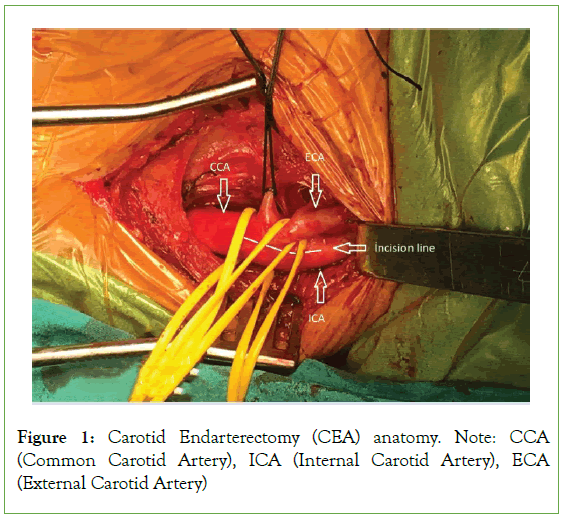
Figure 1: Carotid Endarterectomy (CEA) anatomy. Note: CCA (Common Carotid Artery), ICA (Internal Carotid Artery), ECA (External Carotid Artery)
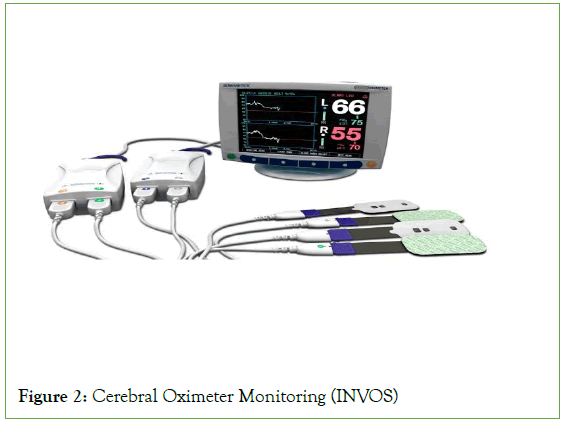
Figure 2: Cerebral Oximeter Monitoring (INVOS)
Advocates have reasoned that among several advantages of locoregional anesthesia, neurologic complications should be less frequent because with LA, the need for shunting can be most accurately assessed (Figure 3) [8]. In addition, patients with coronary and pulmonary diseases should presumably fare better without endotracheal intubation or general anesthesia [11]. Although fairly good results have been achieved with general anesthesia and a variety of monitoring approaches, historically, no single technique has correlated well with the neurological status of conscious patients [11]. All of the direct methods (electroencephalography, evoked potential responses) and indirect methods (carotid stump pressure, transcranial Doppler ultrasound, jugular venous oxygen tension, and cerebral oximetry; INVOS) for detecting cerebral ischemia during carotid cross-clamping have been found at one time or another to lack either sensitivity or specificity when compared with the neurologic status of the awake patient [7]. Electroencephalography, for example, may lead to a 20%-25% higher incidence of intraluminal shunting; the rate of selective shunting in a similar population of conscious patients is reported to be as low as 7% [7]. Conversely, perioperative strokes clearly occur in the absence of electroencephalographic changes [7]. Performing carotid endarterectomy in patients under locoregional anesthesia has also provided invaluable lessons regarding the efficacy of various practices hypothesized to provide cerebral protection, such as induced hypertension, carbon dioxide inhalation, acetazolamide administration, hypothermia, and general anesthesia itself [12].
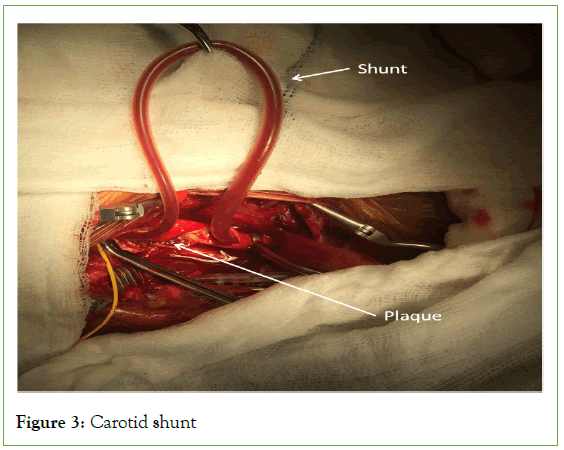
Figure 3: Carotid shunt
Major vascular surgery often necessitates cross-clamping of the blood vessels, exposing patients to the risk of ischemia and reperfusion injury. The mechanisms underlying ischemia-reperfusion injury include free radical formation, mitochondrial failure, and systemic inflammation. Ischemic preconditioning and post conditioning are beneficial in reducing biomarkers of ischemia reperfusion injury, but this has not been shown to translate into a clinical benefit [13,14].
The technique of carotid intraluminal shunting conceivably the ultimate protective mechanism against carotid cross-clamping ischemia, was also originally evaluated and perfected through knowledge acquired from operations on awake patients (Figure 4) [5,12]. Most importantly, operating on conscious patients has allowed accurate differentiation between the various mechanisms of perioperative stroke, especially the distinction between clamping ischemia and nonspecific cerebral embolization [5,12].
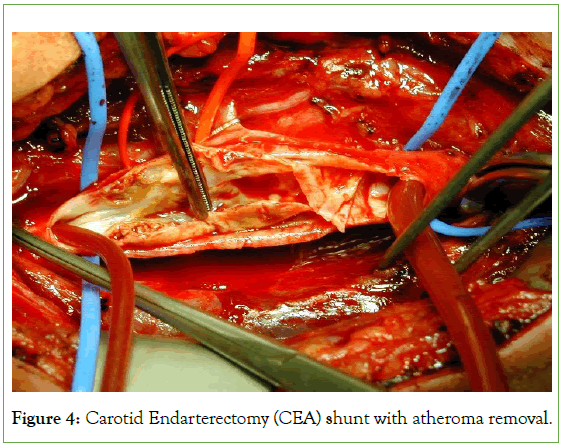
Figure 4: Carotid Endarterectomy (CEA) shunt with atheroma removal.
In the 2019, American Heart Association (AHA) and American Stroke Association (ASA) Guidelines for the Early Management of Acute Ischemic Stroke, emergency or urgent CEA for acute ischemic stroke is classified as Class IIb recommendation with a B-NR level of evidence [13]. To date, there are no unequivocal recommendations regarding the type of anesthesia used to perform CEA in patients during the acute period of ischemic stroke [14].
Locoregional Anesthesia (LA) may have superior advantages compared to general anesthesia [8], such as awake neurological monitoring of patients, early detection of neurological deficits, and reduction in the risk of postoperative myocardial infarction and pulmonary complications [8].
The subject of this article is not new, but the main question of which anesthetic technique, general or locoregional, should be implemented for CEA in terms of patient safety has not been answered [2,3,6]. The objective of this article is to re-examine the results of general versus locoregional anesthesia for CEA in light of the most recent published literature during the last decade and finally suggest a safe anesthetic plan for vascular anesthetists. Currently, the best medical treatment for extracranial carotid artery stenosis is gaining ground against the surgical approach, making the latter’s future role questionable [15]. As there are great risks associated with surgery, patients only benefit from this procedure when perioperative risks are low; therefore, all possible attempts should be made to minimize risks, including the modality of anesthetics [15].
Accordingly, we will point out the effect of anesthetic technique on patient outcomes, especially in terms of morbidity and mortality.
The aim of our study was to analyze the impact of different anesthetic techniques (locoregional versus general) on clinical neurological outcomes (primary endpoint) and mortality rates (secondary endpoint).
Patients and Methods
Over a 10-years period (between June 2013 and June 2023), the hospital medical records of all 2000 consecutive patients who underwent surgery were collected for analysis. A total of 1000 patients under locoregional anesthesia and 1000 patients under general anesthesia were included in the study.
Indications for carotid endarterectomy were asymptomatic carotid artery stenosis of >70% or symptomatic carotid artery stenosis, regardless of the degree of stenosis. Supra-aortic Doppler imaging was performed in all scheduled cases, and the findings were confirmed using Computed Tomography Angiography (CTA). Magnetic Resonance Angiography (MRA) was performed only in symptomatic patients.
The choice of anesthetic method; either LA or GA, was based on patient preference and the general medical condition of the patient following informed explanation and consent during the systemic routinely scheduled pre-anesthetic medical assessment visit.
For the analysis, patients were divided into two groups based on the anesthetic procedure. Comparisons were made with respect to preoperative risk factors, intraoperative events, such as the use of a surgical intraluminal shunt, and postoperative complications. The median operating and carotid cross-clamping times were also recorded. All patients were followed-up perioperatively by a neurologist.
Postoperatively, major stroke was defined as a neurological deficit lasting beyond 30 days, leading to irreversible or slowly reversible prolonged neurological complications.
Minor stroke was defined as any transient focal deficit that did not lead to a handicap, such as combined Transient Ischemic Attack (TIA) and slowly reversible prolonged ischemic neurologic deficit. All patients with new central neurological deficits underwent postoperative cerebral Magnetic Resonance Angiography (MRA). Another complication noted was a significant neck hematoma that required surgical evacuation.
The locoregional anesthetic technique was performed in all patients using a standardized protocol comprising combined ultrasonography-guided superficial and deep cervical plexus blocks (Figure 5). 22-gauge 50 mm stimuplex needle was used. Intravenous (IV) midazolam (2 mg) was administered to all patients before the start of LA. 2 L/min of oxygen was administered via nasal prongs during the surgical procedure. A superficial cervical plexus block was performed just above the carotid bifurcation using 10 ml of lidocaine 2% with 1:200000 adrenaline. A deep cervical plexus block was performed by blocking the second, third, and fourth deep cervical nerve roots (C2, C3, and C4, respectively) with 3 ml of ropivacaine 0.5% for each nerve root. Supplemental infiltration of the ipsilateral mandibular nerve was performed using a mixture of 5 ml of lidocaine 2% and 5 ml of ropivacaine 0.2%. No supplemental xylocaine infiltration was permitted by the surgeon to avoid anesthetizing and obtunding the carotid chemoreceptors to avoid the physiological inhibition of systemic blood pressure autoregulation. Intraoperative continuous intravenous remifentanil infusion was used to improve patient comfort, especially against back muscle contractures and spasms. A typical remifentanil dose range of 0.1-0.2 µg/kg/minute IV infusion was given.
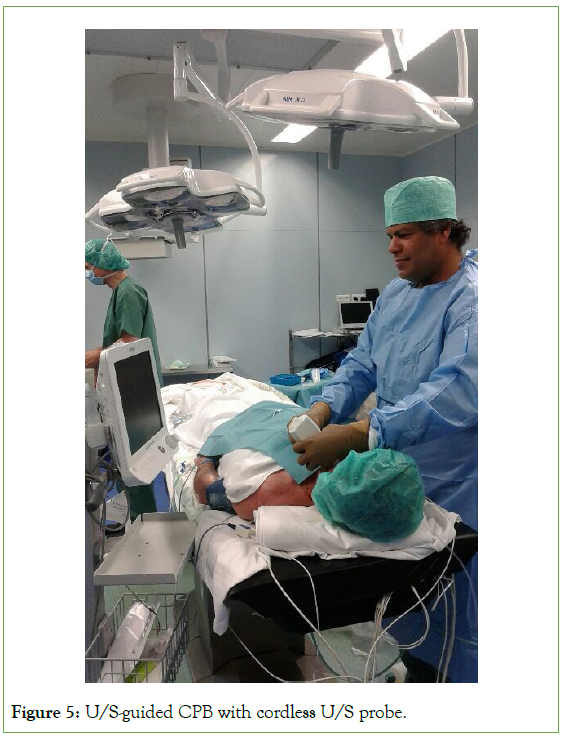
Figure 5: U/S-guided CPB with cordless U/S probe.
The remifentanil dosage can be managed manually according to the patient’s comfort and reflexes, with the aim of allowing the patient to feel comfortable without inhibiting his/her reflexes. Neither benzodiazepines nor hypnotics were permitted during the surgery to avoid inhibiting patient reflexes and consciousness. The aim was to keep the patient conscious, reactive, and comfortable during the procedure. All patients were monitored by standard basic medical monitoring and Cerebral Oximeter Monitoring (INVOSTM 5100C-brain oxygen saturation monitoring with near-infrared spectroscopy) (Figure 6). A radial artery catheter was inserted for invasive arterial blood pressure monitoring under local anesthesia following the end of the LA technique.
Figure 6: INVOSTM 5100C-brain oxygen saturation monitoring.
At the time of carotid cross-clamping, the patient’s neurological status was evaluated by both the patient’s ability to squeeze a giraffe toy to produce identical squeak noise and to answer identity questions (name, date of birth, and address). The duration of the standardized clamping test was 3 minutes. Clinical neurological status was correlated with INVOS findings. A surgical intraluminal carotid shunt was performed if the patient showed initial signs of cerebral ischemia and could not tolerate the carotid cross-clamping test for 3 minutes, coinciding with an ipsilateral reduced trend to more than 30% of the pre-clamping INVOS value.
In the general anesthetic group, a radial artery catheter was inserted first for invasive arterial blood pressure monitoring under local anesthesia, followed by basic arterial blood gas analysis for reference. The patient was endotracheally intubated. GA was induced by intravenous propofol, sufentanil as an opioid analgesic, and cisatracurium as a muscle relaxant. All patients were monitored by standard basic medical monitoring and Cerebral Oximeter Monitoring (INVOSTM 5100C-brain oxygen saturation monitoring with near-infrared spectroscopy). A surgical intraluminal carotid shunt was performed if the INVOS showed an ipsilateral slowing trend to >50% of the pre-clamping INVOS response value.
CEA was performed conventionally using patch closure in all cases, if indicated (Figure 7). Unfractionated heparin (75 IU/kg body weight) was administered intravenously 3 min before carotid cross- clamping and was not routinely reversed.
All patients were admitted to the High-Dependency Unit (HDU) postoperatively. The patients were closely monitored for invasive arterial blood pressure and neurological status. IV antihypertensive medication, for example, nicardipine IV infusion, was used if the postoperative mean arterial blood pressure remained higher than 110 mmHg for more than two hours.
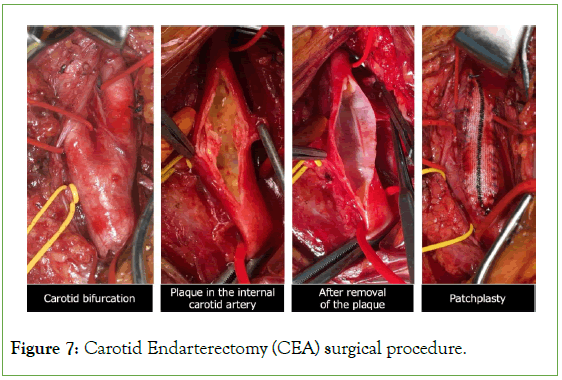
Figure 7: Carotid Endarterectomy (CEA) surgical procedure.
Statistical analysis
Odds Ratios (OR) with 95% Confidence Intervals (CI) were estimated using a multilevel logistic regression model. In the multivariate analysis, the model included age, gender, BMI, smoking history, underlying comorbidities, operative time, and clamping time, which were divided into quartiles. Statistical significance was set at p<0.05. Statistical analysis was performed using StataCorp (2019) (Stata Statistical Software: Release 16; StataCorp LLC, College Station, Texas, USA).
Statistical analysis was performed using the SPSS software (version 15.0; SPSS Inc., Chicago, IL, USA). Comparisons of the groups under consideration were performed using the C2 test for categorical items or t-test for parametric data. The Bonferroni-adjusted P-values as well as the original P-values are given in Tables 1-3. A subgroup analysis was performed with respect to the indication for the operation using the C2 test risk estimate and confidence interval. Stepwise logistic regression analysis was performed to investigate the influence of perioperative factors on postoperative cerebrovascular events and mortality. We considered the Nagelkerke squared R as an estimate of the explained variability. We used the Hosmer- Lemeshow test as a goodness-of-fit check. P-value<0.05 was considered statistically significant (Tables 1-3).
| Patient characteristics | Cervical plexus block (CPB/LA): n°=1000 | General anaesthetics (GA): n°=1000 | Adjusted odd ratio (95% CI) | P-value (<) | All operations: n°=2000 |
|---|---|---|---|---|---|
| Age (mean ± SD) | 61.6 ± 9.1 years | 68.2 ± 7.3 years | 1.025 (0.942-1.115) | 0.001 | 63 ± 8.2 years |
| Male sex (n°) | 621 | 482 | 1103 | ||
| ASA classification | 2.41 ± 0.52 | 2.56 ± 0.51 | 18.122 (2.116-155.241) | 0.008 | 2.5 ± 0.50 |
| CAD | 48.20% | 42.60% | 0.662 (0.076-6.592) | 0.725 | 45.90% |
| AF | 42.60% | 38.10% | 4.913 (0500-48.251) | 0.172 | 39.20% |
| COPD and smoking | 86.90% | 18.60% | 0.999 (0.997-1.000) | 0.001 | 71.20% |
| Diabetes mellitus | 21.60% | 36.50% | 6.425 (1.366-30.215) | 0.019 | 29.80% |
| Advanced PVD | 82.10% | 28.20% | 3.872 (0.903-16.607) | 0.068 | 73.40% |
| Hypertension | 58.40% | 52.30% | 1.199 (0.240-5.991) | 0.825 | 54.60% |
| Advanced CKD | 46.20% | 42.10% | 5.850 (0.737-46.462) | 0.095 | 43.40% |
| Asymptomatic | 21.40% | 32.10% | 0.517 (0.065-4.118) | 0.001 | 28.20% |
| TIA | 38.60% | 26.30% | 0.412 (0.048-2.891) | 0.001 | 32.40% |
| Stroke | 64.80% | 16.20% | 5.155 (1.682-18.681) | 0.001 | 51.60% |
| Baseline NIHSS score | 6.1 ± 3.2 | 5.7 ± 2.9 | 1.548 (0.192-12.507) | 0.682 | 5.8 ± 3.1 |
| Contralateral occlusion | 72.30% | 32.10% | 25.200 (3.168-200.429) | 0.002 | 48.30% |
| Degree of stenosis | |||||
| <70% | 5.90% | 42.30% | 6.865 (1.456-32.315) | 0.001 | 29.60% |
| >70% | 61.40% | 18.60% | 7.850 (0.921-48.524) | 0.001 | 46.20% |
Table 1: Comparison of multivariable predictors of perioperative complications in Locoregional Anesthetics (LA) versus General Anesthetics (GA)
| Variables (median) | Cervical plexus block: (LA) n°=1000 | General anaesthetics: (GA) n°=1000 | Total: n° =2000 | P-value (<) |
|---|---|---|---|---|
| Operative time (min) | 92.31 ± 16.2 | 108.6 ± 12.9 | 96.4 ± 13.8 | 0.001 |
| Clamping time (min) | 23.2 ± 5.4 | 29.6 ± 12.6 | 26.4 ± 8.9 | 0.001 |
| Usage of surgical shunt | 5 | 40 | 45 | 0.001 |
| Inpatient mortality | 0 | 10 (1%) | 10 | |
| HDU stay (day) | 1.1 ± 0.68 | 1.1 ± 0.84 | 1.1 ± 0.71 | 0.001 |
| Hospital stay (day) | 3.2 ± 0.86 | 4.6 ± 0.84 | 3.9 ± 0.71 | 0.001 |
| NIHSS score at discharge | 1.8 ± 2.1 | 1.9 ± 1.8 | 1.8 ± 1.9 | 0.001 |
| Postoperative patient satisfaction | 34.70% | 89.60% | 0.001 | |
| Immediate postoperative hypertension (first 24 hours) | 72.60% | 46.40% | 0.001 | |
| Postoperative Hematoma incidence | 2.10% | 8.20% | 0.001 |
Table 2: Operative details and outcomes of Carotid Endarterectomy (CEA).
| Cervical Plexus Block (CPB/LA): n°=1000 | General Anesthetics: (GA) n°=1000 | Multivariate* OR (95% CI) | Multivariate* P-value (<) | |
|---|---|---|---|---|
| Conversion to general anesthetics (%): | ||||
| Technical failure of CPB/LA | 0 | N/A | ||
| Life-threatening complications | 0 | N/A | ||
| Severe anesthetic complications (%): | ||||
| LA-related Neurological complications | 1 | N/A | ||
| Cardiovascular collapse | 0 | 14 | 1.08 to 4.49 | 0.001 |
| Respiratory distress | 6 | 0 | 1.08 to 3.07 | 0.001 |
| Other anesthetic-related complications (%): | ||||
| Immediate Perioperative CVA/TIA | 3 | 15 | 0.28 to 3.00 | 0.001 |
| Late CVA/TIA (first 30-days postoperative) | 2 | 20 | 0.50 to 3.52 | 0.001 |
| Acute heart failure | 2 | 16 | 1.07 to 4.41 | 0.001 |
| Mortality | 0 | 10 | 1.29 to 4.80 | 0.001 |
| Combined stroke/death (first 30-days postoperative) | 0 | 6 | 1.31 to 3.89 | 0.001 |
Note: (*) Adjusted for gender, age, BMI, smoking, activity of daily living, co-morbidities and hospital-level factors.
Table 3: Complications in carotid surgery under Locoregional Anesthetics (LA/CPB) and General Anesthetics (GA) within 30 days of surgery.
Results
The patient characteristics
In terms of patient demographics, there were slight differences in the clinical characteristics between the two groups. Patients in the LA group were younger (P<0.001) and predominantly male. Younger male patients were more likely to select locoregional anesthetics. The patients in the GA group were older (mean age, 68.2 vs. 61.6 years; P<0.001). We convinced ASA III patients to have locoregional anesthetics due to the high risk of morbidities and mortalities under general anesthesia; However, there was no significant difference between the two anesthetic groups for ASA classification (P<0.008). There were no significant differences between the anesthetic groups for CAD, HTN, CKD, and AF. However, there were differences in COPD and smoking (86.9% in LA vs. 18.6% in GA; P<0.001), diabetes mellitus (21.6% in LA vs. 36.5% in GA; P<0.019), advanced PVD (82.1%in LA vs. 28.2% in GA; P<0.068), TIA (38.6% in LA vs. 26.3% in GA; P<0.001), stroke (64.8% in LA vs. 16.2% in GA; P<0.001), and contralateral carotid occlusion ( 72.3% in LA vs. 32.1% in GA; P<0.002).
Patients with >70% degree of carotid stenosis were 61.4% in the LA group and 18.6% in the GA group (P<0.001); however, in patients with less than 70% degree of carotid stenosis, 42.3% in the GA group versus 5.9% in the LA group (P<0.001).
There were more and significant symptomatic, TIA, and stroke patients in the LA group (P<0.001) and there were more asymptomatic patients in the GA group (32.1% vs. 21.4%, P<0.001). There were more patients with contralateral carotid artery occlusion in the LA group (P<0.002). There were more patients with a degree of carotid stenosis >70% in the LA group (P<0.001) (Table 1). There were no significant differences in baseline NIHSS scores among the patients in either group (P<0.682).
The median operative time, carotid cross-clamping time, HDU stay, and hospital stay were significantly shorter in the LA group (P<0.001) (Table 2).
There was no patient mortality in the LA group versus a 1% mortality rate in the GA group (P<0.001).
The operative details and outcomes of the groups
The median operative time was slightly shorter in the LA group (92.31 min in LA vs. 108.6 min in GA; P<0.001) and median carotid cross-clamping time was shorter under LA (23.2 min in LA vs. 29.6 min in GA; P<0.001).
There was a statistically significant difference in intraluminal shunt use (GA: 40 cases vs. LA: 5 cases).
An intraluminal shunt was used in 40 patients (4.0%) who underwent surgery under general anesthesia. The shunt was placed because the patient demonstrated signs of cerebral ischemia on AIVOS during the carotid cross-clamping test, in contrast to only 5 patients (0.5%) of the operations that were performed with the patient under locoregional anesthesia (Table 2).
Episodes of hypertension were significantly more common in the LA group during the first 24 hours (72.6% in LA vs. 46.4% in GA; P<0.001).
Hematomas requiring surgical evacuation were more commonly observed in the GA group (8.2% in GA vs. 2.1% in LA; P<0.001).
There was no significant difference in the median postoperative HDU stay between the groups (1.1 day; P<0.001).
The median hospital stay was slightly shorter in the LA group (3.2 days in LA vs. 4.6 days in GA; P<0.001).
There was no difference in the median NIHSS score at discharge between the groups (P<0.001).
However, patients in the GA group were significantly more satisfied than in the LA group (89.6% in GA vs. 34.7% in LA; P>0.001).
There was a statistically significant difference in perioperative mortality between the two groups (none in the LA group vs. 10 in the GA group).
The complications of carotid surgery under LA and GA within 30 days of surgery
There was no incidence of converting LA into GA because of the importance of the LA technique with a 100% success rate objective. However, one patient in the LA group experienced mild neurological signs due to local anesthetic toxicity, which was immediately antagonized by IV intralipid, and we did not proceed to convert to GA.
With regard to perioperative neurological complications, there was a significant difference between the groups; 35 patients experienced neurological complications in the GA group versus only 5 patients in the LA group (P<0.001).
15 patients in the GA group experienced immediate postoperative CVA/TIA incident versus only 3 patients in the LA group (95% CI, 0.28-3.0; P<0.001).
Moreover, 20 patients in the GA group experienced CVA/TIA during their first 30-days postoperatively versus only two patients in the LA group (95% CI, 0.50-3.52; P<0.001).
More strokes (2.8% vs. 0.12%) and transient ischemic attacks (5.6% vs. 0.21%), were seen in the GA group. Regression analysis shows that preoperative neurological events, carotid cross-clamping time and the anesthetic technique have a significant impact on postoperative neurological events (Tables 2 and 3). The result should be viewed with caution as the regression analysis, although statistically significant, explains 12.2% of the variability.
14 patients experienced reversible cardiovascular collapse in the GA group versus none patients in LA group (95% CI, 1.08- 4.49; P<0.001) which was managed by simple fluid challenge and vasoactive drugs. However, 6 patients in the LA group experienced respiratory distress following the CPB (95% CI, 1.08-3.07; P<0.001).
16 patients experienced acute heart failure in the GA group versus only 2 patients in the LA group (95% CI, 1.07-4.41; P<0.001) which was managed by inotropes in HDU postoperatively.
The mortality rate in the GA group was significantly higher than that in the LA group; 10 patients died during the first month of surgery in the GA group versus none in the LA group (95% CI, 1.29-4.80; P<0.001).
To compare and quantify the possible influence of all risk factors and operative technique on the occurrence of a cerebrovascular event, a backward stepwise logistic regression analysis was performed (Table 4).
| Significance | Exp (B) | 95% CI (Lower boundary) | 95% CI (upper boundary) | |
|---|---|---|---|---|
| Age | 0.186 | 1.011 | 0.975 | 1.067 |
| Gender | 0.168 | 1.341 | 0.941 | 2.146 |
| Preoperative neurological event | 0.001 | 1.421 | 1.214 | 1.741 |
| Anesthetic technique | 0.003 | 1.984 | 0.894 | 3.412 |
| Operative time | 0.005 | 1.84 | 1.452 | 2.645 |
| Clamping time | 0.002 | 1.084 | 1.024 | 1.064 |
| Occlusive arterial disease | 0.861 | 1.014 | 0.521 | 1.642 |
| Smoker and COPD | 0.286 | 1.148 | 0.698 | 1.894 |
| Chronic kidney dysfunction | 0.721 | 1.124 | 0.487 | 1.678 |
| Coronary artery disease | 0.485 | 0.784 | 0.375 | 1.347 |
| Diabetes mellitus | 0.647 | 0.984 | 0.627 | 1.741 |
| Hypertension | 0.002 | 1.085 | 0.731 | 1.538 |
| Constant | 0 | 0 |
Note: B=Regression coefficient; Exp (B)=Odds Ratio; CI=Confidence Interval.
Table 4: Regression analysis
Hosmer-Lemeshow-Test gave 0.286. The result has to be viewed with caution as the regression analysis, although statistically significant (P<0.001) explains 12.2% (Nagelkerkes R2=0.98) of the variability. Preoperative neurological events (odds ratio 1.4), carotid cross- clamping time (odds ratio 1.08%), and anesthetic technique (odds ratio 1.98%) had a significant impact on postoperative neurological events. In order to predict morbidity and mortality (defined as postoperative mortality or stroke) out of the preoperative and intraoperative variables, the best model (Hosmer-Lemeshow- Significance=0.286) explained 12.2% of the variability. Preoperative cerebrovascular neurological events, carotid cross-clamping time and anesthetic technique were significant predictors of outcome.
Factors affecting perioperative stroke rate
Univariate analysis was performed for the following factors to determine whether they had any significant effect on the perioperative stroke rate: The anesthetic technique, gender, coronary artery disease, diabetes, hypertension, smoking history, COPD, contralateral total occlusion, and preoperative stroke. When this analysis was performed for the entire 10-year series, only a history of preoperative stroke (P<0.002) and carotid cross-clamping time (P<0.002) were found to be associated with perioperative stroke. The use of a general anesthetic was nearly statistically significant in this regard (P<0.003). These three factors were then entered into a multivariate analysis, which identified only a history of preoperative stroke as an independent predictor of perioperative stroke (Table 4). Univariate analysis showed that a history of preoperative stroke (P<0.002) and the use of a general anesthetic (P<0.003) were significantly associated with perioperative stroke. A multivariate analysis was again performed, which identified only a history of preoperative stroke as independently associated with perioperative stroke.
Discussion
Based on our vast experience in anesthetics for three decades, we observed that locoregional anesthetics for the majority of surgical interventions are more advantageous than general anesthetics in terms of superiority in recovery, pain relief, and perioperative hemodynamic stability. The potential benefits of locoregional anesthetics in a wider range of surgical procedures are supported by an overview of several randomized trials [2]. Complications of surgery are also reduced [2]. We introduced the locoregional anesthetic technique to our institution in 2013 to assist in the reduction of morbidities and mortalities following CEA under general anesthesia and effectively offered another realistic anesthetic option to patients. We initially met with resistance from both vascular surgeons and anesthetic colleagues; however, with best-selling methods following a few presentations and showing the evidence, we had the opportunity to endeavor locoregional anesthetics for CEA in our institution. The choice of anesthetic method was based on patient preference and general medical condition. We decided to closely observe our patients and register all the medical data both prospectively and retrospectively to audit our practice and compare it with the general anesthetic group of patients. We collected data from 2000 consecutive patients over a 10-years period, making this one of the largest of its kind being implemented in one institution. Our aim was to investigate whether CEA performed under the two types of anesthetics differed in terms of outcome. One obvious pitfall is inherent in performing a retrospective analysis of clinical data, in that a certain patient selection bias is possible [3,4,6]. Despite the limitations of this retrospective cohort study and the evident lack of randomization, which is regarded as level 2b evidence (EBM principles), a wide range of variables was considered. Our data are based on a rigorous analysis of a large population of patients operated on consecutively over a 10-year period, and prospective data collection was used and standardization of the entire anesthetic, surgical, and monitoring techniques was an important asset of our study; therefore, the power of our study is comparable to level 2a evidence.
Atherosclerotic diseases are widespread in the developed world, with an increasing prevalence in the developing world [16]. The risk factors include smoking, obesity, hypertension, hypercholesterolemia, and diabetes mellitus [16]. Atherosclerotic extracranial carotid artery disease, in the form of carotid intima-media thickening or luminal stenosis, accounts for 24% of ischemic stroke events [16]. Surgery to relieve extracranial carotid atherosclerotic disease and reduce the possibility of ipsilateral stroke due to emboli was first performed at St. Mary’s hospital, London, 1954 [5]. Since then, evidence for the effectiveness of Carotid Endarterectomy (CEA) has accumulated, and CEA has been associated with lower stroke/death rates (<3%) than Carotid Artery Stenting (CAS) [17], although the indications of the latter have expanded during the last decade [17].
The American Heart Association (AHA) guidelines for carotid endarterectomy have laid the foundation for recent practice in carotid surgery [17]. Improvements in endovascular techniques and the development of Carotid Artery Stenting (CAS) have caused a paradigm shift in the treatment of carotid stenosis [17]. Although none of the randomized trials comparing CAS and CEA demonstrated any significant benefit of endovascular stenting over surgery for stroke and death, the classic surgical technique of endarterectomy is still the standard for carotid stenosis treatment [17]. The updated North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the second European Carotid Surgery Trial (ECST-2) are large randomized class 1 studies that have defined current indications for carotid endarterectomy [13,18]. The updated NASCET found that for every six patients treated with CEA, one major stroke would be prevented at 2 years for symptomatic patients with 70%-99% stenosis, whereas the second European Asymptomatic Carotid Surgery Trial (EACST) found that asymptomatic patients may also benefit from the procedure, but only the group with high-grade stenosis (>80% stenosis) [13,18].
What is the ideal anesthetic technique for carotid endarterectomy (general or locoregional)?
In the past 30 years, a plethora of prospective, randomized, controlled trials have compared locoregional and general anesthesia and their effects on outcomes after CEA [2]. It is well established in the literature that locoregional anesthesia improves outcomes in certain aspects after surgery, providing better postoperative analgesia than systemic opioid techniques, reduced blood loss, and a lower risk of thromboembolic events [3,6]. Patient factors interact with the anesthetic technique used to determine the outcome [3,6].
The existing literature related to anesthesia for CEA has consistently reported the effect of the anesthesia technique on mortality [2,3,6]. The first Cochrane review on the subject was published in 1996 and updated in 2013 [4]. Neither the latest Cochrane review nor the GALA trial; the single largest trial available, has shown a statistically significant difference in outcomes between general and local anesthesia for CEA with respect to 30-day incidence of stroke, Myocardial Infarction (MI), and mortality. These studies reported a trend toward lower operative mortality with locoregional anesthesia [2-6]. GALA trial (General Anesthesia versus Local Anesthesia for Carotid Surgery, 2008) compared LA (n=1773) and GA (n=1753) in 3526 patients who underwent CEA [6]. No benefit was observed for either type of anesthesia [5,7]. Patients in the acute stage of ischemic stroke were not included in this study, and CEA was performed for both symptomatic and asymptomatic lesions of the internal carotid artery [6]. A subgroup analysis of GALA study also resulted in decreased neurocognitive performance in the group of general anesthesia [19]. However, the GALA trial has certain limitations rendering its results questionable: (a) it is an underpowered study despite the number of patients included and (b) the precise locoregional anesthetic technique is not tightly controlled [19].
One of the most important advantages of CEA performed under LA is the close monitoring of the neurological status of awake patients [8]. It is extremely reliable to identify the early stages of neurological deficits in patients undergoing LA for CEA [12]. We noticed from our clinical experience that early neurological signs precede the changes in the trends of INVOS; therefore, detecting early neurological deficits in the LA group is more reliable than in patients in the GA group being monitored by INVOS. Early signs of neurological deficits include stupor, confusion, slurring of speech, disorientation, difficulty in holding a grip and squeezing the toy, and difficulty in lifting the contralateral shoulder or arm. Patient cooperation is fundamental during an operation that lasts for up to two hours hence, selection of patients for LA anesthetics is fundamental. Once you identify the early signs of neurological deficits in an awake patient, it is extremely important to proceed to intraluminal surgical shunt outright without hesitation (Figure 8). Indeed, late signs of neurological deficits could be manifested during carotid cross-clamping time exceeding twenty minutes which renders clinically difficult for surgeons to proceed to surgical intraluminal shunt, nevertheless, the neurological deficit signs being manifested late during carotid cross-clamping motivate surgeons to either to perform “declamp-clamp” the carotid artery method to improve the cerebral arterial circulation or to hurry up and end the procedure promptly and eventually declamp the carotid artery. We noticed that after declamping the carotid artery in these situations, patients recovered quickly with no residual neurological deficits.
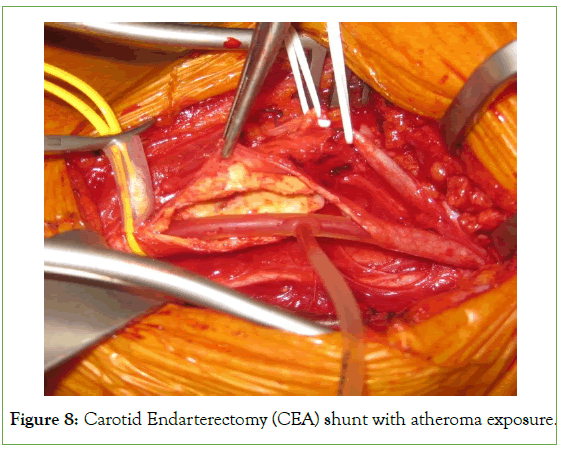
Figure 8: Carotid Endarterectomy (CEA) shunt with atheroma exposure.
The literature describes various reactions to cerebral hypoperfusion during carotid cross-clamping under LA, including depression of consciousness or confusion, psychomotor agitation, aphasia, paresis of the contralateral limbs, and seizures [7]. According to literature, the frequency of conversion from LA to GA in CEA patients ranges from 0.3% to 14.3% [6,20,21]. The main reasons for conversion are inadequate anesthesia, psychomotor agitation of the patient, claustrophobia, local anesthetic toxicity, severe respiratory failure, or protracted surgery due to various reasons [6,19,20,21]. Some surgeons fear that sudden conversion from LA to GA would be shambolic. However, in our study, none of the patients in the LA group were converted to GA due to the meticulous selection of patients and paying maximum attention to details during the execution of the locoregional anesthetic technique. Meanwhile, the perception of pain due to surgical field extension or carotid fascial sheath dissection was improved by additional IV administration of opioids (remifentanil).
We resorted neither to irrigation nor to infiltration of the surgical field with 2% lidocaine solution to avoid obtunding the physiological autoregulatory control of arterial blood pressure which represents one of the advantages of LA however, the development of pain syndrome during mobilization of the carotid arteries is because the carotid sheath is abundantly innervated by the glossopharyngeal and vagus nerves and cannot be anesthetized with cervical plexus blocks [22]. Irrigation of the carotid glomus with a local anesthetic (5 ml of 2% lidocaine solution) is frequently used to suppress unwanted hemodynamic reactions (bradycardia, excessive arterial hypertension, and blood pressure fluctuations); however, we did not use it in our practice. According to Grieff et al., LA was associated with a significantly lower incidence of cranial nerve injury than GA: 1.7% versus 2.9%, respectively (P<0.002) [22].
Of nine reports in the literature that specifically compared locoregional anesthesia with general anesthesia for extracranial carotid surgery, six found no differences in perioperative strokes or death on the basis of anesthetic technique [23]. However, two reports detected an increased rate of perioperative stroke in the general anesthesia group [23]. Three studies reported an increased incidence of cardiopulmonary complications or myocardial infarction in patients who underwent surgery under general anesthesia [15,23]. Meanwhile, Schechter et al. demonstrated a significantly increased incidence of non-neurologic complications among patients who received general anesthesia compared with those who received locoregional anesthesia (12.9% vs. 2.8%) [24]. These findings are in conflict with those of several excellent large series reported in the literature that demonstrated equally low neurologic and cardiopulmonary complication rates with general anesthesia, using various forms of intracerebral monitoring [15,19]. However, there was no significant difference in the incidence of stroke, transient ischemic attacks, or other perioperative complications [15,19].
There was a trend toward higher perioperative stroke and mortality rates in patients who were administered a general anesthetic; however, these patients were seemingly at higher risk, with preoperative stroke as a more common indication for surgery and an increased incidence of contralateral carotid occlusion. Based on these retrospective data, the authors concluded that the anesthetic technique does not have a significant effect on the perioperative outcomes of carotid endarterectomy. This result is predictable; however, the vast experience reflected in this paper is important and creates opportunities for several interesting questions.
With regard to neurological complications, in the largest randomized clinical study, the GALA trial, the incidence of stroke in the LA group was 3.7% (7 of 66 strokes were contralateral to the side of the operation) and in the GA group, it was 4% (15 of 70 strokes were contralateral); the difference between the groups was insignificant [6].
A retrospective prospective study by Lutz et al. on 1341 patients (LA, 465 patients; GA, 876 patients) confirmed that CEA can be performed safely under LA. This may improve the results and lead to better neurological outcomes compared with GA. The risk factor analysis did not reveal any specific risk groups [20].
Orlický et al. compared the incidence of asymptomatic strokes (according to diffusion-weighted magnetic resonance imaging of the brain) in patients undergoing CEA under LA (n=105) and GA (n=105). MRI was performed before surgery and 24 h later. The frequency of newly identified asymptomatic ischemic lesions was significantly lower in the LA group (6.7% vs. 17.1%, P=0.031). Most lesions after LA (71.4%) were associated with embolization, and more than half of the new ischemic injuries after GA (55.5%) were due to cerebral hypoperfusion [25]. The authors believe that such asymptomatic ischemic damage may further impair cognitive function [25].
In our study, there was a significant difference between the two groups in favor of LA in terms of immediate and perioperative TIA and stroke incidence (P<0.001).
Neurological complications were significantly more common in the GA group (3.5% vs. 0.5%), followed by stroke (2.8% vs. 0.12%) and transient ischemic attacks (5.6% vs. 0.21%). Regression analysis shows that preoperative neurological events, carotid cross- clamping time and anesthetic technique had a significant impact on postoperative neurological events. The result should be viewed with caution as the regression analysis, although statistically significant, explained 12.2% of the variability. Clearly, other factors influence the neurological outcome as well. GA has been shown to increase the odds of a postoperative cerebrovascular event by a factor of 2.3.
The most relevant result of our study was the significantly better neurological outcome in favor of locoregional anesthetics.
A retrospective study by Ferrero et al. reported no difference in perioperative neurologic and cardiopulmonary complications between LA and GA in 428 patients [26]. Our experience with a much larger series suggests that LA is safer than CEA. We have shown that the overall complication rate was very low in a large sample of 2000 consecutive cases.
Kasprzak et al. reported no difference in perioperative neurologic and cardiopulmonary complications between both groups [27].
A retrospective study by Liu et al. confirmed that patients receiving LA had significantly lower risks of postoperative unplanned intubation and/or pulmonary resuscitation procedures after carotid endarterectomy than those receiving GA [28].
M.D. Stoneham et al noted the reduced hospital stay in LA, but the similar rate of stroke, compared with GA [29].
Regarding the surgical intraluminal shunt, there was no outright proceeding to routine shunting, and our surgeons did not prefer to proceed to routine shunting, except if it was absolutely indicated either with early signs of neurological deficits in the LA group during the carotid cross-clamping test or sudden changes with INVOS trends in the GA group (Figure 9).
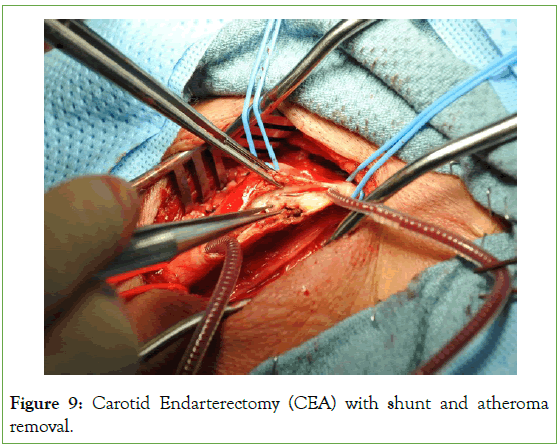
Figure 9: Carotid Endarterectomy (CEA) with shunt and atheroma removal.
Surgeons believe that intraluminal shunts are cumbersome compared to their classic surgical technique; they would prefer to avoid it by any means. Routine shunting is indeed an option, but there may be advantages in selective shunting, such as fewer embolic events due to minimal manipulation of the artery [30,31].
There is always the impression that the more shunt use, the longer the carotid cross-clamping and operating times; however, shunt use has not been shown to be related to neurological complications [30,31]. Routine versus selective intraluminal shunt use and monitoring methods are currently debated [30,31].
In our study, there was a significant difference in shunt use in the LA group (0.5% in LA vs. 4% in GA) (Table 2).
The use of intraluminal surgical shunts is thought to be reduced under LA [17,19]. However Lutz et al. stated in their study that there was no statistically significant difference in shunt use (GA=15.9% vs. LA=13.6%) [20].
Zakirzhanov N. R. et al succeeded in avoiding the use of temporary shunt in patients in hyperacute and acute stages of ischemic stroke [16]. The authors used LA, which, in combination with intraoperative transcranial Doppler and dynamic neuromonitoring in real time, provided an accurate and qualitative assessment of cerebral tolerance to ischemia during carotid cross-clamping [16].
The total operating time and carotid cross-clamping time were shorter under LA (103 min in LA vs. 111 min in GA; P<0.001 and 33 min in LA vs. 39.4 min in GA; P<0.001, respectively) in our study (Table 2).
Lutz et al. confirmed the benefits of performing CEA in awake patients by showing less operative time and HDU stay compared to those operated on under GA [20]. In our study, the median operative and carotid cross-clamping times were significantly shorter in the LA group. The correlation between carotid cross-clamping time and neurological outcome has rarely been reported, but in our series, mean carotid cross-clamping time had a significant impact on neurological outcome in regression analysis (odds ratio 1.084; CI, 1.024-1.064) (Table 4). Other assumed advantages of operating under LA include fewer cardiac and pulmonary complications [21]. In our study, the incidence of Myocardial Infarction (MI) and pneumonia was<1% in the LA group. However, respiratory distress manifested in approximately 0.6% of patients in the LA group due to the most probable ipsilateral block of the phrenic nerve and consequent ipsilateral diaphragmatic paralysis, particularly in COPD patients (P<0.001) (Table 2 and 3).
Regarding myocardial ischemia and infarction, in the largest randomized GALA trial, the incidence of myocardial infarction in the LA group was 0.5% versus 0.2% in the GA group (not significantly different) [5].
Rocha-Neves et al. found that troponin I elevation in the first two days after surgery was detected in 15.3% of patients who underwent CEA under LA. In the long-term follow-up period, patients diagnosed with myocardial injury after CEA under LA remained at a high risk of developing myocardial infarction and other major adverse cardiovascular events [11]. In the general sample, signs of myocardial ischemia were found in 18% of patients operated on under LA and in 23% of patients operated on under GA (the difference was not significant). These data may indicate a preference for the use of LA for CEA in patients with high cardiac risk [11].
Grobben R.B. et al found that troponin I elevation in the first 3 days after surgery was detected in 15.1% of patients who underwent CEA under GA, but myocardial infarction developed in 3.6% in the first 30 days. Thus, clinically confirmed myocardial infarction was observed in only 23.5% of patients with elevated troponin I levels after surgery under GA [32].
Blood Pressure (BP) variability between anesthetic regimes has been reported in several studies [2,3,5,6,8]. Most studies report higher BP variability in patients operated on under GA.
Lutz et al. reported that significantly more patients who underwent surgery under GA had hypertensive events, with systolic blood pressure values greater than 180 mmHg on postoperative day one [20]. However, in our study, the LA group had higher systolic BP in the HDU (72.6% in LA vs. 46.4% in GA, P<0.001) (Table 2).
Bevilacqua et al concluded that there was no difference in postoperative bleeding between the groups [17].
Hussein et al found local hemorrhage significantly more associated with GA in the meta-analysis of the reviewed studies [21]. Surgical evacuation of a hematoma was more commonly performed in patients operated under GA in our study (8.2% in GA vs. 2.1% in LA; P<0.001) (Table 2).
There was no difference in heparin use between the two groups in our study.
Mracek J et al concluded in terms of patient’s satisfaction on 159 patients operated under GA and 30 patients operated under LA were examined [33]. This study concluded that although the level of patient satisfaction was high for both standard anesthesia techniques, satisfaction with anesthesia (AG: 148 patients (93.1%) vs. LA 30 (65.2%); P<0.0001) and preference for the same type of anesthesia in a future operation (AG: 154 patients (96.9%) vs. LA: 28 patients (60.9%); P<0.0001) were significantly higher for GA.
Bevilacqua et al handed to their patients a questionnaire on the first postoperative day, reported higher patient satisfaction with GA([very satisfied: 112 pts. (61.87%); satisfied, 67 (37.01%); dissatisfied, 2 (1.1%); very dissatisfied, 0) [17].
In our study, the overall patient satisfaction was better in the GA group (89.6% in GA vs. 34.7% in LA; P<0.001) (Table 2). Any surgical intervention under LA is a unique and scary experience to patients, and the level of anxiety is heightened during the procedure; benzodiazepines were used during the LA technique at the beginning of the procedure only to reduce anxiety and remifentanil infusion to reduce the discomfort caused by lying on the rigid operating table mattress for a couple of hours during the procedure; however, the study still shows that LA is a difficult experience for patients despite being convinced of its superiority in both outcome and safety compared to general anesthesia.
Conclusion
Carotid endarterectomy under locoregional anesthesia can be performed safely and may lead to better neurological outcomes than with general anesthesia. Risk factor analysis revealed the specific risk groups. The choice of anesthesia should be based on local expertise and the rate of complications.
The choice of anesthetic for use during carotid endarterectomy has remained a matter of debate for more than three decades. The techniques of local and cervical block anesthesia for extracranial carotid surgery have been described and used since before 1962, and the initial reason for using locoregional anesthesia rather than general anesthesia at our institution was to observe the neurological status of the patient during carotid cross-clamping. The observation that a small but significant group of patients was intolerant to carotid cross-clamping, and therefore required a shunt for cerebral protection during carotid endarterectomy, led to a dilemma for surgeons who were more comfortable performing carotid surgery with the patient under general anesthesia. To ensure that unconscious patients do not have a stroke while the operation is being performed, one of two methods can be used: a shunt could be used routinely, or some monitoring technique such as cerebral oximeter monitoring INVOS or Near-infrared spectroscopy could be used to differentiate patients who are at risk for cerebral ischemia so that a shunt could be used selectively. Locoregional anesthesia is an essential tool to aid in the evaluation of various cerebral monitoring techniques and protective measures. However, as experience grows with carotid shunting and cerebral monitoring with the patient under general anesthesia, some investigators concluded that keeping the patient awake was unnecessary in most cases.
However, the selection of patients may be too simplistic to explain the higher complication rate with general anesthesia in recent years. Likewise, to conclude that in earlier years, there was no significant difference between the techniques, may be open to criticism. Because the overall incidence of complications is very low, the outcome of a single case may determine statistical significance in some instances. Without a detailed analysis of the causes of complications, it is hazardous to assume that the choice of anesthetic was the only determining factor.
It is interesting to note that even at institutions that clearly prefer locoregional anesthesia for carotid surgery, the percentage of patients who receive general anesthesia has steadily increased over the past three decades. Of greater concern is the fact that it is in this most recent period that the perioperative stroke rate clearly increases with general anesthesia. It is conceivable that we are currently selecting the highest-risk patients for general anesthesia to an even greater extent than in the earlier years. However, we cannot prove that the general anesthesia patient population as a whole is a higher-risk group than it was in previous years; these patients had an almost identical frequency of preoperative stroke and contralateral total occlusion when compared with patients who underwent surgery more remotely. The perioperative stroke rate for patients in the past 10 years with preoperative stroke who received general anesthesia was somewhat higher than that in patients who received locoregional anesthesia (6.9% vs. 2.3%).
In conclusion, data from RCTs with small sample sizes showed no significant difference between the outcomes of GA and LA use in CEA. When nonrandomized data with much larger sample sizes were also considered, small but significant differences were demonstrated that favored LA. Therefore, larger and more systematically reported studies are required to conclude the debate surrounding the use of GA and LA in CEA. Use of randomization or propensity-matched analysis should be encouraged, and registries should facilitate investigation of this subject. Currently, the choice of anesthetic technique should be considered very carefully to cater to individual patient needs. Surgeons, anesthetists, institutional experience, and comorbidities should be considered in an attempt to optimize outcomes.
To date, there are no unequivocal recommendations regarding the type of anesthesia for performing CEA in patients in the acute period of ischemic stroke. Only one randomized prospective trial could completely determine the small but possibly clinically significant differences between locoregional and general anesthesia.
We conclude from this study that locoregional anesthesia can be safely used for carotid endarterectomy in the vast majority of patients, with good clinical results. The incidence of serious complications associated with the administration of the anesthetic is extremely low. Although the data in our series clearly suggest that locoregional anesthesia may be better than general anesthesia with regard to perioperative stroke, patient selection may account for these differences. However, even if used selectively, we believe that the ability to perform extracranial carotid surgery under locoregional anesthesia is crucial and should be a part of the armamentarium of skills for every vascular anesthetist.
With respect to other significant topics, the operative time, cost, and length of hospital stay were specifically examined in this series, although it has been proven that locoregional anesthesia does not increase operative time. Although we are aware that data regarding these issues are important, especially in the managed care era, there have been too many changes, alluded to above, in the management of extracranial carotid artery stenosis over this lengthy period to make this information meaningful. Most patients are admitted to the morning of surgery, and most are discharged the second morning after surgery; these critical transformations have transpired over the last several years alone; therefore, we presume that during the next few years, locoregional anesthesia for CAE will be an effective and reliable clinical background for developing Enhanced Recovery Programs (ERPs) and fast-track surgery for endovascular and carotid endarterectomy.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Author's Contributions
All authors were involved in the conception and design of the trial and final approval of the final manuscript.
All relevant data are within the paper and its Supporting Information files.
References
- Finn C, Giambrone AE, Gialdini G, Delgado D, Baradaran H, Kamel H, et al. The association between carotid artery atherosclerosis and silent brain infarction: A systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2017; 26(7):1594-1601.
[Crossref] [Google Scholar] [PubMed]
- Licker M. Regional or general anaesthesia for carotid endarterectomy: Does it matter?. Eur J Anaesthesiol. 2016; 33(4):241-243.
[Crossref] [Google Scholar] [PubMed]
- Hopkins PM. Does regional anaesthesia improve outcome?. Br J Anaesth. 2015; 115(suppl_2):ii26-33.
[Crossref] [Google Scholar] [PubMed]
- Vaniyapong T, Chongruksut W, Rerkasem K. Local versus general anaesthesia for carotid endarterectomy. Cochrane Database Syst Rev. 2013; 12:CD000126.
[Crossref] [Google Scholar] [PubMed]
- Gough MJ, Bodenham A, Horrocks M, Colam B, Lewis SC, Rothwell PM, et al. GALA: An international multicentre randomised trial comparing general anaesthesia versus local anaesthesia for carotid surgery. Trials. 2008; 9(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Pasin L, Nardelli P, Landoni G, Cornero G, Magrin S, Tshomba Y, et al. Examination of regional anesthesia for carotid endarterectomy. J Vasc Surg. 2015; 62(3):631-634.
[Crossref] [Google Scholar] [PubMed]
- Dellaretti M, de Vasconcelos LT, Dourado J, de Souza RF, Fontoura RR, de Sousa AA. Locoregional anesthesia for carotid endarterectomy: Identification of patients with intolerance to cross-clamping. World Neurosurg. 2016; 87:61-64.
[Crossref] [Google Scholar] [PubMed]
- Rocha-Neves J, Pereira-Macedo J, Ferreira A, Dias-Neto M, Andrade JP, Mansilha AA. Impact of intraoperative neurologic deficits in carotid endarterectomy under regional anesthesia. Scand Cardiovasc J. 2021; 55(3):180-186.
[Crossref] [Google Scholar] [PubMed]
- Guay J. The GALA trial: Answers it gives, answers it does not. Lancet. 2008; 372(9656):2092-2093.
[Crossref] [Google Scholar] [PubMed]
- Jonsson M, Lindström D, Wanhainen A, Gidlund KD, Gillgren P. Near infrared spectroscopy as a predictor for shunt requirement during carotid endarterectomy. Eur J Vasc Endovasc Surg. 2017; 53(6):783-791.
[Crossref] [Google Scholar] [PubMed]
- Malik OS, Brovman EY, Urman RD. The use of regional or local anesthesia for carotid endarterectomies may reduce blood loss and pulmonary complications. J Cardiothorac Vasc Anesth. 2019; 33(4):935-942.
[Crossref] [Google Scholar] [PubMed]
- Chongruksut W, Vaniyapong T, Rerkasem K. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev. 2014; 6(6):CD000190.
[Crossref] [Google Scholar] [PubMed]
- Cheng SF, van Velzen TJ, Gregson J, Richards T, Jäger HR, Simister R, et al. The 2nd European Carotid Surgery Trial (ECST-2): Rationale and protocol for a randomised clinical trial comparing immediate revascularisation versus optimised medical therapy alone in patients with symptomatic and asymptomatic carotid stenosis at low to intermediate risk of stroke. Trials. 2022; 23(1):606.
[Crossref] [Google Scholar] [PubMed]
- Zakirzhanov NR, Komarov RN, Khalilov IG, Baiazova NI, Evseeva VV. Comparative analysis of safety of carotid endarterectomy performed in acutest and acute periods of ischaemic stroke. Angiol Sosud Khir. 2021; 27(1):97-106.
[Crossref] [Google Scholar] [PubMed]
- Bevilacqua S, Ticozzelli G, Orso M, Alba G, Capoccia L, Cappelli A, et al. Anesthetic management of carotid endarterectomy: An update from Italian guidelines. J Anesth Analg Crit Care. 2022; 2(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016; 118(4):535-546.
[Crossref] [Google Scholar] [PubMed]
- Müller MD, Lyrer P, Brown MM, Bonati LH. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Cochrane Database Syst Rev. 2020; 2(2):CD000515.
[Crossref] [Google Scholar] [PubMed]
- AMIN HP. Carotid endarterectomy for symptomatic moderate carotid stenosis. The NASCET Trial. 2016:283-288.
- Weber CF, Friedl H, Hueppe M, Hintereder G, Schmitz-Rixen T, Zwissler B, et al. Impact of general versus local anesthesia on early postoperative cognitive dysfunction following carotid endarterectomy: GALA study subgroup analysis. World J Surg. 2009; 33:1526-1532.
[Crossref] [Google Scholar] [PubMed]
- Lutz HJ, Michael R, Gahl B, Savolainen H. Local versus general anaesthesia for carotid endarterectomy–improving the gold standard?. Eur J Vasc Endovasc Surg. 2008; 36(2):145-149.
[Crossref] [Google Scholar] [PubMed]
- Hussain AS, Mullard A, Oppat WF, Nolan KD. Increased resource utilization and overall morbidity are associated with general versus regional anesthesia for carotid endarterectomy in data collected by the Michigan Surgical Quality Collaborative. J Vasc Surg. 2017; 66(3):802-809.
[Crossref] [Google Scholar] [PubMed]
- Grieff AN, Dombrovskiy V, Beckerman W, Ventarola D, Truong H, Huntress L, et al. Anesthesia type is associated with decreased cranial nerve injury in carotid endarterectomy. Ann Vasc Surg. 2021; 70:318-325.
[Crossref] [Google Scholar] [PubMed]
- Harky A, Chan JS, Kot TK, Sanli D, Rahimli R, Belamaric Z, et al. General anesthesia versus local anesthesia in carotid endarterectomy: A systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2020; 34(1):219-234.
[Crossref] [Google Scholar] [PubMed]
- Schechter MA, Shortell CK, Scarborough JE. Regional versus general anesthesia for carotid endarterectomy: The American college of surgeon’s national surgical quality improvement program perspective. Surgery. 2012; 152(3):309-314.
[Crossref] [Google Scholar] [PubMed]
- Orlicky M, Hrbac T, Sames M, Vachata P, Hejcl A, Otahal D, et al. Anesthesia type determines risk of cerebral infarction after carotid endarterectomy. J Vasc Surg. 2019; 70(1):138-147.
[Crossref] [Google Scholar] [PubMed]
- Ferrero E, Ferri M, Viazzo A, Ferrero M, Gaggiano A, Berardi G, et al. Carotid endarterectomy: Comparison between general and local anesthesia. Revision of our experience with 428 consecutive cases. Ann Vasc Surg. 2010; 24(8):1034-1037.
[Crossref] [Google Scholar] [PubMed]
- Kasprzak, Altmeppen, Angerer, Mann, Mackh, Topel. General versus locoregional anesthesia in carotid surgery: A prospective randomised trial. Vasa. 2006; 35(4):232-238.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Martinez-Wilson H, Neuman MD, Elkassabany N, Ochroch EA. Outcome of carotid endarterectomy after regional anesthesia versus general anesthesia-a retrospective study using two independent databases. Transl Perioper Pain Med. 2014; 1(2):14.
[Google Scholar] [PubMed]
- Stoneham MD, Stamou D, Mason J. Regional anaesthesia for carotid endarterectomy. Br J Anaesth. 2015; 114(3):372-383.
[Crossref] [Google Scholar] [PubMed]
- AbuRahma AF, Mousa AY, Stone PA. Shunting during carotid endarterectomy. J Vasc Surg. 2011; 54(5):1502-1510.
[Crossref] [Google Scholar] [PubMed]
- Kim TY, Choi JB, Kim KH, Kim MH, Shin BS, Park HK. Routine shunting is safe and reliable for cerebral perfusion during carotid endarterectomy in symptomatic carotid stenosis. Korean J Thorac Cardiovasc Surg. 2012; 45(2):95.
[Crossref] [Google Scholar] [PubMed]
- Grobben RB, Vrijenhoek JE, Nathoe HM, Den Ruijter HM, Van Waes JA, Peelen LM, et al. Clinical relevance of cardiac troponin assessment in patients undergoing carotid endarterectomy. Eur J Vasc Endovasc Surg. 2016; 51(4):473-480.
[Crossref] [Google Scholar] [PubMed]
- Mracek J, Kletecka J, Holeckova I, Dostal J, Mrackova J, Mork J, et al. Patient satisfaction with general versus local anesthesia during carotid endarterectomy. J Neurol Surg A Cent Eur Neurosurg. 2019; 80(05):341-344.
[Crossref] [Google Scholar] [PubMed]
Citation: Bayoumy RE, Cesarini M, Besnard M, Casanova A (2023) A Comparison of Locoregional versus General Anesthesia in Patients Undergoing Carotid Endarterectomy: A Retrospective Single-Center Study. J Surg Anesth. 7:215.
Copyright: © 2023 Bayoumy RE, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
