Indexed In
- Academic Journals Database
- Open J Gate
- Genamics JournalSeek
- JournalTOCs
- China National Knowledge Infrastructure (CNKI)
- Scimago
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- MIAR
- University Grants Commission
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Google Scholar
Useful Links
Share This Page
Open Access Journals
- Agri and Aquaculture
- Biochemistry
- Bioinformatics & Systems Biology
- Business & Management
- Chemistry
- Clinical Sciences
- Engineering
- Food & Nutrition
- General Science
- Genetics & Molecular Biology
- Immunology & Microbiology
- Medical Sciences
- Neuroscience & Psychology
- Nursing & Health Care
- Pharmaceutical Sciences
Research Article - (2022) Volume 0, Issue 0
A Comparison between GeneXpert Testing and the Berlin-Charite Diagnostic Protocol for the Detection of SARS-CoV-2 in a Cohort of Mexican Patients
Eduardo Becerril-Vargas1*, Gabriel Cojuc-Konigsberg1, Gastón Becherano-Razon1, Ángel Sánchez-Tinajero2, Yessica Saraí Velazco-García1, José Arturo Martínez-Orozco2, Andrea Iraís Delgado-Cueva2, Danna Patricia Ruiz-Santillán2, Antonio- Juárez Etzael1, Rodríguez-Sánchez Víctor Manu1, Valencia-Trujillo Daniel1, García Colín María del Carmen1, Mujica- Sánchez Mario1, Mireles-Dávalos Christian Daniel1, Montiel Molina Yamil Baruch1, Daniel de la Rosa Martinez3 and Diana Vilar-Compte3Department of Infectious Disease, National Institute of Respiratory Diseases (INER), Mexico City, Mexico
2Department of Infectious Disease, National Institute of Cancer, Mexico City, Mexico
Received: 16-Dec-2021, Manuscript No. JVV-21-15119; Editor assigned: 20-Dec-2021, Pre QC No. JVV-21-15119; Reviewed: 03-Jan-2022, QC No. JVV-21-15119; Revised: 10-Jan-2022, Manuscript No. JVV-21-15119; Published: 17-Jan-2022, DOI: 10.35248/2157-7560.22.S17.005
Abstract
Since the beginning of the SARS-CoV-2 pandemic healthcare professionals have found it necessary to generate diagnostic methods for the disease that are easy to use, reliable, and accessible.
The Berlin-Charité protocol has been one of the most recommended methods for detecting SARS-CoV-2 from the onset of the pandemic. However, new diagnostic techniques such as GeneXpert have been developed and proven to be efficient, fast, and easy to use to detect infected patients.
The purpose of this study, conducted at the National Institute for Respiratory Diseases in Mexico, was to compare the diagnostic performance of the Berlin-Charité protocol and GeneXpert for the detection of SARS-CoV-2, evaluating a cohort of 135 Mexican patients. For statistical analysis, sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratios for each assay were calculated.
The diagnostic parameters for GeneXpert were found to be 100% in both sensitivity and specificity. The Berlin- Charité protocol performance had a sensitivity of 72% and specificity of 100%.
With this study, it can be concluded that the diagnosis of SARS-CoV-2 infection through GeneXpert was 29% more specific than the Berlin protocol.
Keywords
SARS-CoV-2; GeneXpert; Berlin-Charité protocol
Introduction
Coronavirus Disease 2019 (COVID-19) has caused drastic changes in the way the world is conceived: In social behaviors and relationships, in the economy, and particularly in the way health services are provided. Information about the virus and management protocols has been evolving constantly, urging healthcare professionals to develop and find efficient ways to control the pandemic.
As of April 6, 2021, according to the World Health Organization (WHO), more than a year after the first case was reported in the world the virus responsible for this pathology had caused the death of about 2.8 million people around the world and infected around 130 million people. However, as the pandemic has progressed and having at some point exceeded 7% of the global mortality rate, as of April 2021 the WHO had reported a mortality rate of around 2%of the 219 countries where cases have been reported, the Americas and Europe are the most affected with 48% and 34% of cases globally, respectively [1].
In Mexico, according to the General Directorate of Epidemiology (DGE), more than 200,000 deaths and 2.25 million confirmed cases have been reported, which corresponds approximately to 2% of the world's cases. Mexico City (CDMX) is where most COVID-19 cases and deaths have been reported in the country [2].
The Real-Time Polymerase Chain Reaction (RT-PCR) is considered, so far, as the gold standard for the diagnosis and confirmation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) according to the WHO. This assay can detect and measure minute amounts of nucleic acids in different sample types. However, there have been some documented cases where discharged COVID-19 patients later tested positive for SARS-CoV-2, and according to a systematic review carried out by Chenxi Li and his group, variables such as mismatches between primers, probes, and target sequences, as well as the large number of genetic variations shown by viruses, compromise the diagnostic performance of this test, making it very specific but not as sensitive [3,4].
The use of diverse reagents, protocols, and analysis methods for RT- PCR assay can alter the results of the test making it not as reliable as was thought at the beginning of the pandemic. Several studies have proposed to standardize the assay procedure around the world to get more reliable results. The Cycle threshold value (Ct) is the indicator for detectable viral RNA in the amplification process of the RT-PCR. Ct value varies between different samples and the system used to process the sample, however, for the diagnosis of COVID-19 the Ct should range between 16.9 and 38.8 [5].
It is important to emphasize that in the initial stages of the SARS- CoV-2 infection, having a normal chest Computed Tomography (CT) scan does not rule out the diagnosis, making it necessary to perform a microbiological test [6]. However, in the opposite scenario, when the clinical and tomographic data are suggestive of COVID-19, negative RT-PCR should not exclude the diagnosis [7].
On January 17, 2020, the World Health Organization (WHO) published an update of the approved protocols to perform laboratory tests, and on February 1 of the same year, the Pan American Health Organization (PAHO) adopted the protocol developed by the Charité Hospital, Berlin, Germany, as the gold standard for the detection of SARS-CoV-2 [8].
The Berlin-Charité protocol integrates the diagnosis of SARS-CoV-2 employing three independent PCR. The first PCR consists of the amplification and detection of the gene E, a region of the viral envelope. This gene is present in different betacoronavirus and is not specific to SARS-CoV-2. If the test results were positive, then the second confirmatory PCR is performed looking to amplify a specific region of the SARS-CoV-2, located at the RNA-dependent RNA polymerase (RdRp). If this yields a positive result, the third PCR should be done with a human control gene, usually ribonuclease P to verify the viability of the sample. The process for making the diagnosis of the disease following this protocol can delay the diagnosis and treatment of patients and uses a high amount of reagents that could be used in other tests [9].
Single cartridge-based assays are another diagnostic method used for SARS-CoV-2, but the accessibility to these methods is limited in this situation, mainly due to higher costs and low global availability [10]. Cartridge-based assays allow the diagnosis of patients in a short period of time even if they are asymptomatic, which helps in the epidemiological management of the pandemic [11].
The GeneXpert Dx instrument is performed with the recently released Xpert Xpress SARS-CoV-2 assay, a rapid, RT-PCR test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 in upper respiratory specimens. It requires a sample load of 300 μL, has a detection limit of 250 copies per mL, and the whole test takes 45 min [12]. The viral envelope E gene and the nucleocapsid N2 gene are the targets in this assay. The overall sample sensitivity and specificity is 100%, with no cross-reactivity reported with other coronaviruses [10].
This study aims to compare the Berlin-Charité diagnostic protocol and the GeneXpert tool for the detection of SARS-CoV-2.
Materials and Methods
A prospective, comparative and observational study was conducted from May 1st to May 31th, 2020. Medical information was collected through the evaluation of medical records, laboratories, and ancillary studies of patients with suggestive symptoms of COVID-19 at the National Institute of Respiratory Diseases INER in Mexico City. All patients were ≥ 18 years with clinical and/ or epidemiological data consistent of COVID-19. All patients underwent RT-PCR testing for the confirmatory diagnosis of SARS-CoV-2 infection and chest CT.
The patients were classified in two groups, either healthy or confirmed. The healthy group was defined as asymptomatic or paucisymptomatic patients without Computed Tomography (CT) scan abnormalities and a negative RT-PCR test according to Xpert Xpress SARS-CoV-2 protocol Patients were categorized as confirmed if they had symptoms or tomographic abnormalities that suggested SARS- CoV-2 infection, and a positive RT-PCR using Xpert Xpress SARS-CoV-2.
Of the 135 evaluated patients, 102 were submitted to both GeneXpert and Berlin protocol testing. 52 patients were in the asymptomatic patients and 50 in the confirmed group.
Results are presented through descriptive statistics; frequencies, percentages or averages with standard deviations were used for quantitative variables, whereas qualitative variables were expressed through frequencies and percentages. The Kolmogorov-Smirnov test was used for the normality test when the (n) was greater than or equal to 30, otherwise, the Shapiro-Wilk test was used. The analysis of the diagnostic performance of the tests, values of sensitivity, specificity, positive predictive value, negative predictive value, and probability ratio of confirmed and healthy patients, were determined with the elaboration of contingency tables. Statistical analysis was performed using IBM SPSS 27.
Results
Sociodemographic data
A total of 135 patients were included: 51% (68/135) showed symptoms consistent with COVID-19 and 49% (67/135) were asymptomatic. Mean age was 45 years ± 14.83. The male/female ratio was 2: 1. 39% (40/135) of the people included in our study had at least one comorbidity. DM2 and malignant neoplasms were the most frequent comorbidities found in the patients included in the analysis (Table 1). Of the 135 cases, 44.4% (60/135) were health-care personnel (Table 2). Nurses and physicians were seen most frequently, 55% (33/605) and 23% (14/60) respectively (Table 2).
| Comorbidities | N | %N=135 |
|---|---|---|
| DM2 | 22 | 16% |
| Malignant neoplasms | 18 | 13% |
| HBP | 15 | 11% |
| Asthma | 3 | 2% |
| Other cardiopathies | 2 | 1% |
| Lung diseases | 2 | 1% |
| Others | 18 | 13% |
Table 1: Frequency of comorbidities in the sample.
| Occupation | N | %N=135 |
|---|---|---|
| Healthcare personnel | 60 | 44% |
| Nursing staff | 33 | 55% |
| Medical staff | 14 | 23% |
| Respiratory therapy technicians | 5 | 8% |
| Other | 8 | 13% |
| Merchandisers | 21 | 16% |
| Drivers | 7 | 5% |
| Housewives | 5 | 4% |
| Unemployed | 5 | 4% |
| Others | 37 | 27% |
Table 2: Occupation of patients included for analysis.
Seventy three percent of patients were symptomatic (58/79). Symptoms most frequently reported were cough as the main symptom, followed by fever in 63% and headache in 62%, (49/79). Only 5(6%) patients presented conjunctivitis. Tachypnea and chest pain were relatively frequent, occurring in up to 20% of patients (Table 3).
| Symptoms | Total (N=79) |
|---|---|
| Cough | 73% (58/79) |
| Headache | 62% (49/79) |
| Fever | 63% (50/79) |
| Dyspnea | 60% (47/79) |
| Asthenia | 58% (46/79) |
| Myalgias | 48% (39/79) |
| Arthralgias | 42% (33/79) |
| Odynophagia | 35% (28/79) |
| Chills | 28% (22/79) |
| Rhinorrhea | 23% (18/79) |
| Irritability | 23% (18/79) |
| Chest pain | 20% (16/79) |
| Tachypnea | 20% (17/79) |
| Diarrhea | 19% (15/79) |
| Other gastrointestinal symptoms | 11% (9/79) |
| Conjunctivitis | 6% (5/79) |
Table 3: Most common symptoms.
Diagnostic performance result
The sensitivity, specificity, PPV, and NPV of GeneXpert for the detection of SARS-CoV-2 were found to be 100% in all determinations. The positive probability ratio was greater than 100 and the negative probability ratio was 0.
The Berlin-Charité protocol performance had a sensitivity of 100% specificity of 72%, PPV 60%, NPV 100%, positive probability ratio of 3.57, and negative probability ratio of 0 (Table 4).
| Number of patients | Xpert Xpress SARS-CoV-2 | Sensibilidad (%) | Especificidad (%) | VPP a (%) | VPN a (%) | Positive likelihood ratio | Negative likelihood ratio | |
|---|---|---|---|---|---|---|---|---|
| Positives | Negatives | |||||||
| With COVID-19 | 50 | 0 | 100 | 100 | 100 | 100 | >100 | 0 |
| Without COVID-19 | 0 | 52 | ||||||
| Number of patients | Berlin-Charité Protocol | Sensibilidad (%) | Especificidad (%) | VPP a (%) | VPN a (%) | Positive likelihood ratio | Negative likelihood ratio | |
| Positives | Negatives | |||||||
| With COVID-19 | 30 | 20 | 100 | 72 | 100 | 76 | 3.57 | 0.4 |
| Without COVID-19 | 0 | 52 | ||||||
Table 4: Most common symptoms.
The Median Ct value of positive samples according to Xpert Xpress SARS-CoV-2 was 27.43 ± 9.19. This was lower in positive tests using Berlin-Charité protocol, 25.28 ± 6.90, compared 2to negative results 31.2 ± 11.46, but the difference was not statistically significant (p=0.20).
Discussion
COVID-19 confirmed case is that in which there is a positive RT-PCR or viral culture, regardless of clinical manifestations or epidemiological context. Although not confirmatory, suggestive clinical data is enough to suspect the presence of the disease and start providing care to patients [13].
As the gold standard to detect viral RNA in SARS-CoV-2 infections, RT-PCR can have false-negative results due to many circumstances, such as inadequate pre-analytic processing, genetic variability, and lack of sensitivity for low amplification samples. Especially, false negatives are due to low viral loads [4,14,15]. It is important to consider the high Crossing threshold (Ct) when performing RT- PCR for SARS-CoV-2, since many patients are positive for one gene and negative for the other, and these patients cannot be ruled out as positives [16].
A negative RT-PCR for SARS-CoV-2 does not necessarily rule out an infection. As proven by Assaad, et al. approximately 80% of cancer patients with clinical characteristics consistent with COVID-19 had a negative test, populations in whom it is imperative to consider other diagnostic options in the face of symptoms suggestive of COVID-19 and the poor outcomes they can have [17].
Wang, et al. has stated that neither chest CT nor RT-PCR tests are accurate enough for the diagnosis of COVID-19 infection by themselves [18]. In some cases, even with a negative RT-PCR, a positive chest CT could be enough to diagnose COVID-19, having a greater sensitivity [7,19].
New tests, such as molecular assays and panels, could be even more practical than RT-PCR to diagnose and treat COVID-19 in an early manner. As Li, et al. has mentioned it is important to combine and improve testing methods, since efficient laboratory diagnoses are key to controlling the pandemic [4].
Many molecular assays and diagnostic protocols have been proposed to specify the identification of SARS-CoV-2, performing excellently [20,21]. Mostafa, et al. demonstrated that the seven most common automated molecular assays can detect the virus accurately, showing that particularly GeneXpert could detect every replicate at a lower nucleocapsid concentration [21].
In our cohort, every statistical parameter evaluated for GeneXpert proved to be 100%, further proving the efficacy of this diagnostic tool. However, we found that the Berlin-Charité protocol had a lower specificity and positive predictive value than GeneXpert.
Contrasting our results with Vaz, et al. where a similar analysis was made, the concordance between diagnostic methods varied. In the study conducted by Vaz, et al. the Charité-Berlin protocol and Xpert Xpress system had a complete concordance in identifying SARS-CoV-2, as opposed to ours, where the GeneXpert method performed better. Nevertheless, the GeneXpert automated amplification assay had lower variability when evaluating particular genes [22].
In our study, as well as in Etievant, et al. the Berlin-Charité protocol included many false positives, with a need for further analysis [20].
GeneXpert is a high yield test to diagnose COVID-19, as is the Berlin-Charité protocol, the latter with some noteworthy considerations. As exposed in a published letter by Dramé and collaborators, it is worth noting that there is a need to evaluate gold standard diagnostic studies realistically, without assuming their infallibility [23].
Some limitations to our study are that molecular assay kits are useful in laboratories, but they might not be optimal for immediate patient evaluation and follow-up [24]. Moreover, false negatives may occur due to the intricacies of COVID-19, such as the variable incubation period. Besides, the emergency state declaration in Mexico did not allow for the complete evaluation of patients over a long period, as well as following up on their results.
Further studies are needed to demonstrate the reasons for false positives in the Berlin-Charité protocol and the accuracy of GeneXpert in larger populations. Additionally, as the pandemic evolves and new promising techniques emerge, their efficacy should be evaluated and compared to the current standards of practice (Figure 1).
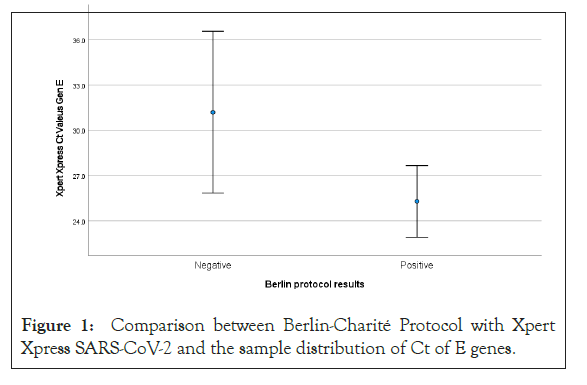
Figure 1: Comparison between Berlin-Charité Protocol with Xpert Xpress SARS-CoV-2 and the sample distribution of Ct of E genes.
Conclusion
When comparing the diagnostic performance of GeneXpert against the Berlin-Charité protocol, it can be concluded that GeneXpert is 29% more specific than the Berlin-Charité protocol. Also, the PPV is 40% lower in the latter, which translates into low usefulness for the detection of SARS-CoV-2 in the context of a pandemic, conveying the need to consider other alternatives to reach an accurate and reliable diagnosis.
Although RT-PCR testing is still considered worldwide as the benchmark par excellence to confirm infection by SARS-CoV-2, it could be worth thoroughly exploring its benefits and reviewing to what extent this test has changed the diagnostic ability in the management of the pandemic, thereby opening up considerations to new diagnostic methods such as the ones presented in this study.
Broader and deeper studies are needed as a matter of fact to corroborate with greater scientific weight the findings exposed above. Nevertheless, this study aims to awaken the curiosity of the scientific community for new confirmatory methods for the diagnosis of COVID-19 and to rethink the true utility of those hitherto deemed to be "gold standards.”
REFERENCES
- Weekly epidemiological update on COVID-19-6 April 2021. World Health Organization (WHO). 2021.
- Federal Health Secretariat. Coronavirus COVID-19 Daily Technical Release. Mexico City: General Directorate of Epidemiology. 2021.
- Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323(15):1502-1503.
[Crossref], [Google Scholar]
- Li C, Zhao C, Bao J, Tang B, Wang Y, Gu B. Laboratory diagnosis of coronavirus disease-2019 (COVID-19). Clinica Chimica Acta. 2020;510:35-46.
[Crossref], [Google Scholar], [PubMed]
- Sule WF, Oluwayelu DO. Real-time RT-PCR for COVID-19 diagnosis: Challenges and prospects. Pan Afr Med J. 2020;35(Suppl 2).
[Crossref], [Google Scholar], [PubMed]
- Yang W, Yan F. Patients with RT-PCR-confirmed COVID-19 and normal chest CT. Radiology. 2020;295(2):E3.
[Crossref], [Google Scholar], [PubMed]
- Pakdemirli E, Mandalia U, Monib S. Positive chest CT features in patients with COVID-19 pneumonia and negative real-time polymerase chain reaction test. Cureus. 2020;12(8):e9942.
[Crossref], [Google Scholar], [PubMed]
- Laboratory guidelines for detection and diagnosis of the novel Corona Virus (2019-nCoV) Infection. Pan American Health Organization (PAHO). 2020.
- Rua KP, Correa JF, Aguilar-Jiménez W, Ayala CA, Rugeles MT, Zuluaga AF. Validation of a duplex PCR technique using the gen E and RNase P for the diagnosis of SARS-CoV-2. Enferm Infecc Microbiol Clin. 2021.
[Crossref], [Google Scholar], [PubMed]
- Goldenberger D, Leuzinger K, Sogaard K, Gosert R, Roloff T, Naegele K, et al. Brief validation of the novel GeneXpert Xpress SARS-CoV-2 PCR assay. J Virol Method. 2020;284:113925.
[Crossref], [Google Scholar], [PubMed]
- Oâ??Connell S, Conlan C, Reidy M, Stack C, Mulgrew A, Baruah J. The impact of point-of-care testing for influenza A and B on patient flow and management in a medical assessment unit of a general hospital. BMC Res Note. 2020;13(1):1-5.
[Crossref], [Google Scholar], [PubMed]
- FDA. Xpert® Xpress SARS-CoV-2. Sunnyville: FDA. 2021:1-26.
- Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020;40(5):351-360.
[Crossref], [Google Scholar], [PubMed]
- Falasca F, Sciandra I, Di Carlo D, Gentile M, Deales A, Antonelli G, et al. Detection of SARS-CoV N2 gene: Very low amounts of viral RNA or false positive?. J Clin Virol. 2020;133:104660.
[Crossref], [Google Scholar], [PubMed]
- Ãlvarez-DÃaz DA, Franco-Muñoz C, Laiton-Donato K, Usme-Ciro JA, Franco-Sierra ND, Flórez-Sánchez AC, et al. Molecular analysis of several in-house rRT-PCR protocols for SARS-CoV-2 detection in the context of genetic variability of the virus in Colombia. Infect Genet Evol. 2020;84:104390.
[Crossref], [Google Scholar], [PubMed]
- Khoshchehreh M, Wald-Dickler N, Holtom P, Butler-Wu S. A needle in the haystack? Assessing the significance of Envelope (E) gene-negative, Nucleocapsid (N2) gene-positive SARS-CoV-2 detection by the Cepheid Xpert Xpress SARS-COV-2 assay. J Clin Virol. 2020;133:104683.
[Crossref], [Google Scholar], [PubMed]
- Assaad S, Avrillon V, Fournier ML, Mastroianni B, Russias B, Swalduz A, et al. The high mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135: 251-259.
[Crossref], [Google Scholar], [PubMed]
- Wang Y, Hou H, Wang W, Wang W. Combination of CT and RT-PCR in the screening or diagnosis of COVID-19. J Glob Health. 2020;10(1): 1-2.
[Crossref], [Google Scholar], [PubMed]
- Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiol. 2020;296(2): E115-E117.
[Crossref], [Google Scholar], [PubMed]
- Etievant S, Bal A, Escuret V, Brengel-Pesce K, Bouscambert M, Cheynet V, et al. Performance assessment of SARS-CoV-2 PCR assays developed by WHO referral laboratories. J Clin Med. 2020;9(6):1871.
[Crossref], [Google Scholar], [PubMed]
- Mostafa HH, Hardick J, Morehead E, Miller JA, Gaydos CA, Manabe YC. Comparison of the analytical sensitivity of seven commonly used commercial SARS-CoV-2 automated molecular assays. J Clin Virol. 2020;130:104578.
[Crossref], [Google Scholar], [PubMed]
- Vaz SN, Santana DS, Martins E, Wang WK, Brites C. Validation of the GeneXpert Xpress SARS-CoV-2 PCR assay using saliva as biological specimen. Braz J Infect Dis. 2021;25.
[Crossref], [Google Scholar], [PubMed]
- Dramé M, Tabue Teguo M, Proye E, Hequet F, Hentzien M, Kanagaratnam L, et al. Should RT-PCR be considered a gold standard in the diagnosis of COVIDâ?19?. J Med Virol. 2020;92(11):2312-2313.
[Crossref], [Google Scholar], [PubMed]
- Yüce M, Filiztekin E, �zkaya K. COVID-19 diagnosis: A review of current methods. Biosens Bioelectron. 2021;172:112752.
[Crossref], [Google Scholar], [PubMed]
Citation: Becerril-Vargas E, Cojuc-Konigsberg G, Becherano-Razon G, Sanchez-Tinajero A, Velazco-Garcia YS, Martinez-Orozco JA, et al. (2022) A Comparison between Genexpert Testing and the Berlin-Charite Diagnostic Protocol for the Detection of SARS-CoV-2 in a Cohort of Mexican Patients. J Vaccines Vaccin. S17: 005.
Copyright: © 2022 Becerril-Vargas E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

